In vitro cell-based assays are a suitable and economic method for assessing test compound toxicity, particularly in drug discovery research programs. A usual workflow involves test compound titration, application to cells, and incubation for a number of days. All cytotoxicity assay-specific liquid handling tasks were carried out by using the CyBio® FeliX (Analytik Jena) instrument as a stand-alone, completely automated system.

Effects of the test compounds were evaluated by using reagents developed to quantify biomarkers of interest. Put simply, a compound or treatment that brings forth a cytotoxic effect does so by causing remarkable morphological variations, negatively impacting replication rate or resulting in a decrease in feasible cell count. Information obtained from in vitro approaches is a vital precursor to identify potential toxic problems. In mechanistic cytotoxicity profiling, distinctive biomarkers are used to report the health status of cells. In this article, a panel of three assays was employed to evaluate biomarkers related to variations in cell number, apoptosis, and membrane integrity (cytotoxicity).
When employed in combination, these biomarkers can help differentiate between populations of cells that are apoptotic, necrotic, or growth-arrested. Table 1 describes the assays used for this study.
Table 1. Description of the Promega cell health assays used for automated cytotoxicity profiling in this study
| Assay |
Determination |
Assay Determination Description |
| CellTox™ Green |
Cytotoxicity or compromised cell membrane |
Nonlytic reagent comprised of a DNA binding dye that is permeable to cells with a compromised cell membrane. The dye becomes highly fluorescent when bound to DNA, and the fluorescent signal is proportional to the number of dead cells in a sample. The dye is conveniently added to the cell suspension at the time of cell plating allowing cytotoxicity to be monitored over time. The nonlytic reagent can be multiplexed with other spectrally distinct fluorescent dyes or luminescent assays. TM375 |
| CellTiter-Glo® 2.0 Assay |
Cell number or viability |
Lytic reagent that quantifies the ATP present in a cell population using a luminescent firefly luciferase reaction. Luminescent signal is proportional to ATP content and indicates the number of viable cells. TM403 |
| Caspase-Glo® 3/7 Assay |
Apoptosis |
Lytic luminescent assay that quantifies caspase-3/7 activity, enzymes active in cells undergoing latestage apoptosis. A DEVD-proluciferin substrate is cleaved by caspase-3/7 to generate free luciferin that is quantified in a firefly luciferase reaction. Luminescent signal is proportional to caspase-3/7 activity and indicates cells undergoing apoptosis. TB 323 |
The CellTox™ Green Assay was multiplexed with the Caspase-Glo® 3/7 Assay or CellTiter-Glo® 2.0 to reduce sample processing and to conserve cell samples. The detection chemistries of Caspase-Glo® 3/7 and CellTiter-Glo® 2.0 are incompatible. Thus, duplicate samples were assembled to separately evaluate apoptosis and cell viability. For this profiling workflow, frozen, thaw-and-use cells provide a suitable option when compared to cells from continuous culture. Thaw-and-use cells such as these are prepared and plated directly after thawing, not requiring any prior cell culture activities. The biological performance of cultured cells can be affected by confluence and passage number, and the use of thaw-and-use cells can overcome these challenges.
The profiling workflow is made more efficient by using laboratory automation for all experimental steps. This article explains an automated serial titration and application of test compound(s) to an assay plate, as well as the addition of reagents and cells. Combining automation guarantees reliable experimental setup by avoiding user-to-user variability in pipetting and improves laboratory efficiency.
For this application, Frozen Instant HepG2 Cells (Cell Culture Service), a human liver cell line, a GloMax® Discover multimode detection system (Promega) for detection of fluorescent and luminescent assay signals, and a CyBio® Felix instrument for all liquid handling steps have been employed. In order to adopt this automated profiling process, minimum time and attention devoted to experimental steps are adequate. The automated process allows up to eight compounds to be tested in quadruplicate in 384-well microplate format. When time course studies are preferred, duplicate plates can be assembled.
Challenge
To develop an automated workflow for determining the mechanism of chemical-mediated cytotoxicity.
Solution
The flexible and compact CyBio® FeliX pipetting system was used to carry out all liquid handling steps of the cytotoxicity assay.
Materials and Methods
Samples and Reagents
- DMSO (Sigma Aldrich, Cat.# D8418)
- Panobinostat (Selleckchem, Cat.# S-1030), 10 mM in DMSO
- Methotrexate (Sigma Aldrich, Cat.# M9929), 10 mM in DMSO
- Nocodazole (Sigma Aldrich, Cat.# M1404), 10 mM in DMSO
- Tamoxifen Citrate (Sigma Aldrich, T9262), 10 mM in DMSO
- Staurosporine (EMD Millipore, Cat. #569397), 10 mM in DMSO
- Cisplatin (Sigma Aldrich, Cat.# P4394), 10 mM in DMSO
- Terfenadine (Sigma Aldrich, Cat.# T9652), 10 mM in DMSO
- CellTox™ Green Express Cytotoxicity Assay (Promega, Cat.#G8731)
- CellTiter-Glo® 2.0 Assay (Promega, Cat.# G9242)
- Cellgro DMEM, 1X with 4.5 g/glucose; L-glutamine and sodium pyruvate (Corning Cat.# 10-012-CV) supplemented with 10% FBS
- Caspase-Glo® 3/7 Assay (Promega, Cat.# G8093)
- EMEM with L-Glutamine (ATCC Cat.# 30-2003) supplemented with 10% FBS and 5% DMSO
- EMEM with L-Glutamine (ATCC Cat.# 30-2003) supplemented with 10% FBS and 1X Penicillin/Streptomycin
- Costar® v-bottom polystyrene 96-well assay plate (Corning, Cat. #3896)
- 8-row partitioned reservoir (Seahorse Bio Cat.# 201282-100)
- White 384-well assay plate (Greiner bio one Cat.# 781080)
- 384 HH, 4X control, pyramid bottom reservoir (Seahorse Bio Cat.# 201300-100)
- Bortezomib (LC Laboratories, Cat.# B-1408), 10 mM in DMSO
- Frozen Instant Cells, HepG2 (Cell Culture Service, ID 89)
- 96 LP pyramid bottom reservoir (Seahorse Bio Cat.#201254-100)
Instrumentation
CyBio® FeliX Automated Liquid Handler
Including 12 deck positions on two levels, the CyBio® Felix automated liquid handler enables liquid transfer of multiple reagents between tube racks, multi-well plates, and reservoirs in a small footprint of 650 mm x 450 mm (WxD; Figure 1).

Figure 1. The CyBio® FeliX pipetting station. The two-level deck design of the CyBio® FeliX possesses a small footprint while providing sufficient deck positions for reagents and lab ware consumables. Users may customize the instrument configuration to meet their specific needs.
An 8-channel liquid handling adapter and a 96-channel pipetting head (250 μL) were used. The 96-channel head allows simultaneous processing of 96-well and 384-well multi-well plates when using pre-racked CyBio® RoboTipTrays. The 8-channel adapter enables pipetting into single wells and columns. Rapid dispensing eliminates concerns of cell settling in the source reservoir and reduces the exposure time of cells to the environment. The switching of liquid handling adapters and the loading of the pipette tips were carried out by the pipetting head without manual intervention, enabling walk-away automation of the assays.
GloMax® Discover Multimode Detection System
The GloMax® Discover system incorporates high-performance luminescence, filtered luminescence, UV-Visible absorbance, fluorescence, FRET, BRET, and kinetic measurement potentials (Figure 2). The built-in tablet PC incorporates intuitive software with fast drag-and-drop protocol creation. Quick-read functions and over 40 preloaded and validated protocols enable quick and effortless data generation.

Figure 2. The Promega GloMax® Discover System. The GloMax® Discover is a multimode reader that accepts plate types ranging from 6-well to 384-well multi-well plates. The instrument is operated by using integrated tablet PC containing enhanced software with an intuitive graphical interface. Quick-read functions and preloaded detection protocols enable fast data generation. Drag and drop functionality expedites the creation of customized detection protocols.
Plate Layouts and Reservoir Configuration
For compound titration, a 96-well v-bottom plate (Corning) has been used as v-shaped wells help reduce dead volume. Compounds were added into columns 1 and 6 of the dilution plate (Figure 3) and the 8-channel adapter has been used to add medium to rest of the columns and to carry out the serial five-point titration for compounds in each column. The 96-channel adapter is subsequently used to multi-dispense titrated compound to the 384-well assay plate. An eight-row reagent reservoir helped save deck space by letting CellTiter-Glo® as well as Caspase-Glo® 3/7 detection reagents to be placed in the same source location (Figure 3). The 96-channel adapter with CyBio® RoboTipTray 96/250 μL was used to shift both solutions to the assay plate at the same time.
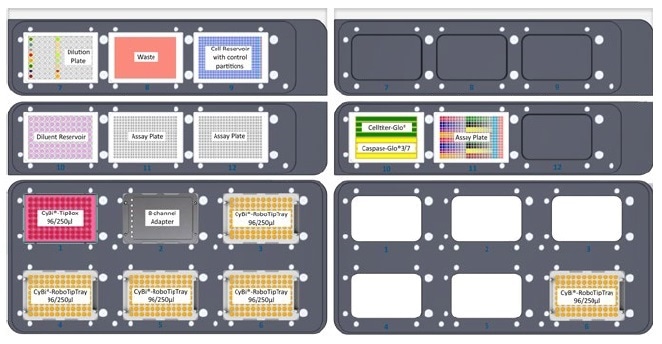
Figure 3. Plate layouts and cell reservoir configuration. Layouts of upper and lower decks. Note position and internal layout of cell reservoir with control partitions, the 96-well compound dilution plate, and the eight-row reagent reservoir containing Cell Titer-Glo® and Caspase-Glo® reagents.
Plate Setup
- HepG2 cells were thawed and suspended in EMEM + 10% FBS + 1X streptomycin/penicillin at a density of 250,000 cells/mL. CellTox™ Green reagent was mixed with the medium at a dilution ratio of 1:800; the overall dye dilution at the end of assay assembly is 1:1000.
- The test compounds were diluted in EMEM + 10% FBS to 5X concentrations ranging between 125 and 500 μM; Manually ordering of 100 μL of each diluted compound to columns 1 and 6 of a 96-well plate (Figure 3) was performed, and the plate was positioned above the deck of the CyBio® Felix at position 7.
- Diluent containing EMEM + 5% DMSO + 10% FBS was transferred into a 96-well low-profile pyramid-bottom reservoir.
- The cell suspension was transferred into the chief partition of a 384-well 4X control pyramid-bottom reservoir. A no-cell control containing EMEM + 10% FBS + CellTox™ Green reagent was filled into the control partitions of the reservoir (see Figure 3), and the reservoir was kept at position 9.
- Two 384-well assay plates were mounted on the instrument deck at positions 11 and 12, and a CyBi®-TipBox 96/250 μL, an 8-channel liquid handling adapter, and four CyBi®-RoboTipTrays 96/ 250 μL were added to the bottom deck.
- The automated protocol was started, directing the CyBio® Felix to carry out the following liquid handling operations: 20 μL of cell suspension and no-cell controls were poured into each well of the assay plates. A 5-point 1:10 serial titration of the compounds was carried out in 5% DMSO in EMEM + 10% FBS diluent. And then, 5 μL of each drug titration was added in quadruplicate to the cells in the assay plate. There were no cells in columns 23 and 24 of the assay plate to rationalize background signal (Figure 4).
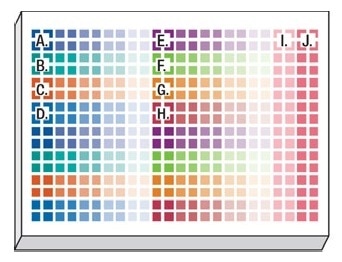
Figure 4. Assay plate layout showing titrated compounds and controls. Using 384-well plates, we replicated the same compounds and dispense pattern in Rows A-H and Rows I-P. Key: A = bortezo-mib, B = cisplatin, C = methotrexate, D = nocodazole, E = panobinostat, F = staurosporine, G = terfenadine, H = tamoxifen, I = 1 % DMSO vehicle control, J = no-cell control.
Reagent Addition and Signal Detection
- A cell culture incubator was used for incubating the assay plates for 24 or 48 hours, at a temperature of 37 °C and with 5% CO2. After each time point, the temperature of the assay plate was lowered to ambient temperature for 15 minutes and then shaken for 1 minute on an offline electromagnetic shaker (Union Scientific).
- Next, the plate was kept in the GloMax® Discover to compute fluorescence changes from the CellTox™ Green dye with the help of the blue filter pair [470 nm (excitation)/500–550 nm (emission)].
- 5 mL of CellTiter-Glo® 2.0 reagent was filled to rows 1 to 4 of an 8-row partitioned reservoir, and 5 mL Caspase-Glo® 3/7 reagent was filled to the remaining rows (Figure 3). The assay plate and the reagent reservoir were positioned on the top deck of the CyBio® FeliX instrument.
- The CyBio® FeliX dispensed 25 μL of both reagents to the assay plate at the same time
- The plate was shaken for two minutes and incubated at ambient temperature for an hour
- Subsequent to the incubation, luminescence was measured on the GloMax® Discover. Using the GraphPad Prism® software, results for cell viability (CellTiter-Glo® 2.0 Assay), cytotoxicity (CellTox™ Green Express Assay), and apoptosis (Caspase-Glo® 3/7 Assay) were plotted.
Results and Discussion
Three toxic compounds that are identified to cause unique and specific mechanisms of cell death were used to evaluate three mechanistic cytotoxicity profiles. Panobinostat is a non-selective histone deacetylase inhibitor and exhibits a hallmark dose-dependent rise in caspase-3/7 activity with an equivalent drop in the cell viability marker, ATP. In this study, a small rise in the cytotoxicity biomarker has been identified, denoting that these apoptotic cells are starting to experience secondary necrosis (Figure 5, Panel A).
Tamoxifen is an estrogen receptor antagonist, and in this study, it offers example results of cells going through secondary necrosis at the time of detection, which took place after the induction of apoptosis. As observed in Figure 5, Panel B, at 24 hours, the highest concentration of tamoxifen tested (100 μM) generated a cytotoxic profile including membrane integrity variations with intense adverse impact on cell viability. However, some caspase-3/7 biomarker is identified.
Prior studies have demonstrated that this concentration of tamoxifen does stimulate apoptosis within a few hours of treatment (1); however, the biomarker decay causes the amount of caspase activity detected to decrease eventually.
The low-level caspase activity detection, together with membrane breakdown and a huge drop in cell viability, implies that apoptosis did take place much earlier and that the 24-hour time point is not within the window of maximum detection for apoptosis. Methotrexate is a dihydrofolate reductase inhibitor that eventually inhibits the production of RNA and DNA. As observed at 24-hour exposure in Figure 5, Panel C, the caspase and membrane integrity biomarkers remain the same without any change, while the cell viability biomarker exhibits a strong dose-dependent effect. Since the cell membrane biomarker remains the same, the resulting reduction in signal compared to control indicates an anti-proliferative, instead of cytotoxic effect.
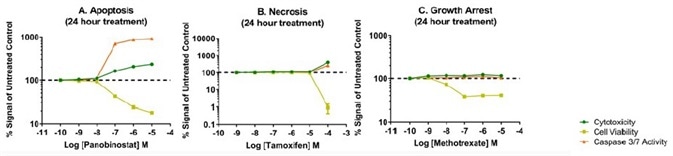
Figure 5. Representative mechanistic cytotoxicity profiles demonstrating (Panel A) apoptosis, (Panel B) necrosis, and (Panel C) growth arrest at 24 hours post treatment. We subtracted medium only control assay background from the experimental results and plotted data as fold change relative to the untreated control on the assay plate.
Exposure time is a vital parameter in the evaluation of mechanistic cytotoxicity. Illustrated in Figure 6, are the effects of nocodazole, an inhibitor of microtubule polymerization. None of the assayed biomarkers showed change after 24 hours post treatment, implying that cells remain viable in culture. A biologically different biomarker profile is seen 48 hours post-treatment. Dose-dependent reductions in cell viability with corresponding increases in caspase-3/7 activity and cytotoxicity are observed, signifying that cells are developing through apoptosis and secondary necrosis. Thus, concentrating testing efforts on 24 hours only could result in the false inference that the test compound does not impact cell health, while constant exposure indicates a cytotoxic effect.
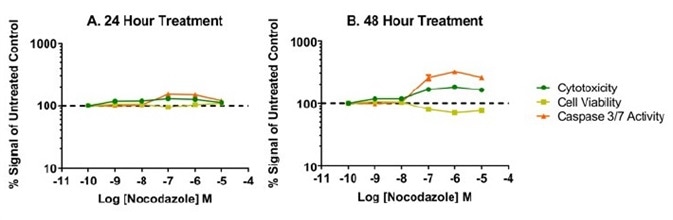
Figure 6. Time and dose-dependent effects of nocodazole, an antimitotic agent that disrupts microtubule formation. Overall low %CV was achieved across each titration of compound as shown by small error bars on our data graphs. The %CV was typically 5% or lower among the four replicates and reflects precise liquid handling for all additions to the well. It was found that the 8-channel adapter performed accurate titration of test compound as supported by the expected potency o response with our model test compounds.
Conclusion
This article reports the successful implementation of an automated approach for differentiating apoptosis, necrosis, and growth arrest. It involved the use of thaw-and-use HepG2 cells as biological model system, the CyBio® FeliX for liquid manipulation, fluorescent assays and commercial bioluminescent to evaluate cytotoxicity, and GloMax® Discover for readout. In order to assess if the chemical treatment induced apoptosis, necrosis, or growth arrest, three biomarkers were employed.
Representative compound profiles demonstrated predicted data patterns for each unique mechanism of toxicity: panobinostat for apoptosis, tamoxifen for secondary necrosis with some apoptosis, and methotrexate for growth arrest. Furthermore, it shows the significance of compound exposure time when determining the mechanism of cytotoxicity (1). The standard 24-hour exposure time might be too early to identify some apoptotic mechanisms (for example, the nocodazole assays).
On the whole, the CyBio® FeliX liquid handler has been found to be an ideal platform for performing the mechanistic cytotoxicity experiments mentioned in this article. The liquid handler enabled test compounds to be consecutively titrated and applied to the cells; it is a flexible, competitively priced instrument that can perform required assay setup and reagent delivery steps of a profiling workflow. The inclusion of thaw-and-use cells offers a reproducible source of cells and eliminates the trouble of devoting time to cell culture, and the use of the CyBio® FeliX liquid handler eliminated the troublesome and more variable manual liquid handling.
Such an automated workflow offers walk-away convenience to scientists while producing reliable and reproducible results. The coupling of homogeneous “add-mix-measure” cell health assays and the GloMax® Discover from Promega guarantees sensitive and optimized detection for significant cell cytotoxicity profiling data.
References
- Riss, T.L. and Moravec, R.A. (2004) Use of multiple assay endpoints to investigate the effects of incubation time, dose of toxin, and plating density in cell-based cytotoxicity assays Assay Drug Dev. Technol. 2, 51–62.
About Analytik Jena US
Analytik Jena is a provider of instruments and products in the areas of analytical measuring technology and life science. Its portfolio includes the most modern analytical technology and complete systems for bioanalytical applications in the life science area.
Comprehensive laboratory software management and information systems (LIMS), service offerings, as well as device-specific consumables and disposables, such as reagents or plastic articles, complete the Group’s extensive range of products.
About Life Science
The Life Science product area demonstrates the biotechnological competence of Analytik Jena AG. We provide a wide product spectrum for automated total, as well as individual solutions for molecular diagnostics. Our products are focused to offer you a quality and the reproducibility of your laboratory results.
This will surely ease your daily work and speed up your work processes in a certain way. All together we support you through the complete process of the lab work. Besides we offer customized solutions and are able to adapt our products to your needs. Automated high-throughput screening systems for the pharmaceutical sector are also part of this segment’s extensive portfolio.
About Analytical Instrumentation
Analytik Jena has a long tradition in developing high-performance precision analytical systems which dates back to the inventions made by Ernst Abbe and Carl Zeiss. We have grown to become one of the most innovative manufacturers of analytical measuring technology worldwide.
Our business unit Analytical Instrumentation offers excellent competencies in the fields of optical spectroscopy, sum parameters and elemental analysis. Being proud of our core competency we grant all our customers a long-term warranty of 10 years for our high-performance optics.
About Lab Automation
With more than 25 years of market experience, Analytik Jena with its CyBio® Product Line is a leading provider for high quality liquid handling and automation technologies. In the pharmaceutical and life science industries, our products enjoy the highest reputation for precision, reliability, robustness and simplicity.
Moreover, the Automation Team designs, produces and installs fully automated systems tailored to our clients' application, throughput and capacity requirements. From stand-alone CyBio® Well up to fully customized robotic systems we handle your compounds, biomolecules and cells with great care.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.