In this article, Analytik Jena showcases the technology it developed to drastically reduce the time it takes to prepare sample protein assays with liquid handling.
Simple WesternTM Jess is a high throughput western blot technique with exactly quantifiable data. Unfortunately, sample preparation and Simple WesternTM are laborious processes susceptible to errors.
The CyBio FeliX was employed to conduct the liquid handling phases of the sample protein assay and the Simple WesternTM plate preparation.
Automated solution for Simple WesternTM Jess — a high-throughput western blotting technique
The Simple-WesternTM Jess is a capillary-based automated western blot system that can run 24 samples, from generation to results, in around five hours. This throughput is advantageous over conventional western blot instruments, which typically run 10 to 20 samples, require 4 to 5 hours of bench time, and take 24 to 48 hours to yield results.
Automating the protein separation and immunodetection process removes the fallible stages of conventional western blotting, and the related software processes the quantifiable data rapidly. At Aurelia Bioscience, the Simple-WesternTM Jess is a fundamental technique in a range of drug discovery methodologies.
Therefore, the CyBio FeliX liquid handling system was utilized to streamline the Simple-WesternTM set-up procedure.
Automating the liquid handling process of Jess plate preparation would enhance data reproducibility, provide greater flexibility to researchers by eliminating manual tasks in the workflow, and enable less experienced users to utilize the technology.
THAL-SNS-032, an extensively studied Proteolysis-targeting chimera (PROTAC) molecule and targeted degrader of cyclin-dependent kinase 9 (CDK9), was utilized to highlight the capabilities of CyBio FeliX and to compare the outcomes with previously obtained data presented in the Protein-SimpleTM webinar “Advancing Targeted Protein Degradation Research with Automated Western Blotting.”
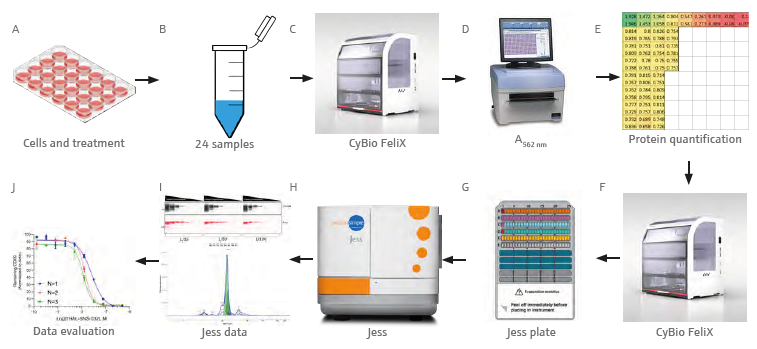
Figure 1. Graphical workflow demonstrating the analytical process. Transfer and lysis of treated cells (A) in culture to tubes (B). BCA assay set up by the CyBio FeliX (C) and absorbance measurement at 562 nm (D). Evaluation of the quantification data (E), Jess plate set up by the CyBio FeliX (F, G), Jess run (H), data acquisition (I) and analysis (J). Image Credit: Analytik Jena US
Materials and methods
Samples and reagents
- Pierce BCA protein assay kit – Thermo Fisher #23227 (Reagents B and A are mixed at a 1:50 ratio before use)
- BCA reagent A, 500 ml
- BCA reagent B, 25 ml
- 12-230kDa Jess/Wes separation module 8x25 capillary cartridges – Bio-Techne #SM-W004
- Anti-Rabbit Detection module for Jess/Wes – Bio-Techne #DM-001
- Anti-Mouse Secondary NIR antibody – Bio-Techne #043-821
- MV-4-11 Cells – ATCC #CRL-9591
- RPMI-1640 – Thermo Fisher #21875-034
- Fetal Bovine Serum – Corning #35-079-CV
- PBS tablets – Thermo Fisher 18912014 (1 tablet per 500 mL sterile water)
- 1x Complete RIPA lysis buffer:
- RIPA – Thermo Fisher #89900
- Magnesium Chloride – Merck #M1028-100 ml (FAC 2mM)
- Benzonase clease – Merck #E1014-25KU (0.5 μL per mL)
- Pierce Protease Inhibitor Tablets, EDTA-free – Thermo Fisher #A32965 (1 tablet per 50 mL lysis buffer)
- THAL-SNS-032 – Tocris #6532
- Anti-CDK9 antibody – Cell Signaling Technology #2316
- Anti-β-actin antibody – Bio-Techne #MAB8929
Instrumentation and consumables
- CyBio FeliX Basic Unit with Enclosure (Analytik Jena GmbH) (OL5015-24-100)
- CyBio FeliX CHOICE Head (OL3316-14-250)
- 8-channel CHOICE Adapter; 10-1000 μL (OL3316-14-330)
- 8-channel CHOICE Adapter; 1-50 μL (OL3316-14-332)
- 2 x Adapter 24 tubes, passive cooling function (844-00136-0)
- CyBio Waste box (844-00430-0)
- CyBio TipBox 96/250 μL Sterile (OL3811-25-637-S)
- CyBio TipBox 96/50 μL Non-sterile (OL3811-25-535-N)
- Adapter 96, passive cooling function (844-00135-0)
- 2 x Height adapter 40 mm (844-00445-0)
- 12-channel Reservoir Plate – Alphalabs #LW9400
- Simple-Western Jess
- Perkin-Elmer EnSpire – Reading plate at 562 nm
- 1.5 mL Eppendorf Tubes – SLS #E0030123328
- PCR tube strip and lids – Alphalabs #LW2535
- 24-well plate – Thermo Fisher #930186
- Polystyrene clear 384-well plate – Greiner #781101
- Plate Seals – Alphalabs #LW2770
- Bio-Rad MyCycler thermal cycler
- Thermo Scientific Megafuge 8R + M10 microplate swinging bucket rotor
- 37 °C incubator – Stuart Scientific incubator SI 18
Cell treatment and lysate preparation
MV-4-11 cells are seeded in a 24-well sterile polystyrene plate and incubated at 37 °C with 5% CO2 and humidity.
Table 1. BSA standard curve with concentrations. Source: Analytik Jena US
| Standard curve tube label |
Concentration of BSA (mg/mL) |
| A |
2.0 |
| B |
1.5 |
| C |
1.0 |
| D |
0.75 |
| E |
0.5 |
| F |
0.25 |
| G |
0.125 |
| H |
0.025 |
| I |
0.0 |
The cells are treated with serially diluted SNS-THAL-032 in technical duplicate and then collected in 1.5 mL tubes. After being washed in PBS, the cells are lysed in 1x complete RIPA lysis buffer, incubated for 30 minutes on ice, and then clarified at 17,000 × g at 4 °C for 10 minutes.
Bicinchoninic acid (BCA) assay (CyBio FeliX)
A standard curve was created from samples with known concentrations of BSA (2.0-0.025 mg/mL) in an appropriate buffer. The BCA assay determined the protein concentration of a cell lysate by extrapolating unknown values from this standard curve. This assay used BSA concentrations to simultaneously determine the protein concentrations from 24 distinct samples.
Before the BCA assay was run, CyBio FeliX prepared the dilution series of protein standards to be utilized in multiple BCA assays of a separate protocol. The CyBio FeliX performs the assay by adhering to the plate template and the instructions outlined below:
- 6 μL of each protein standard is added twice across wells A1-A9 and B1-B9 of a polystyrene 384-well plate (highlighted in Figure 2B).
- A 1/3 protein sample dilution is conducted in the well utilizing a single channel and 50 μL tips as outlined below:
- 4 μL of lysis buffer is added to a designated well of the 384-well plate in duplicate.
- A new tip is picked up and 2 μL of sample is then added to each of the duplicate wells (resulting in a volume of 6 μL in each well).
- 50 μL of pre-mixed BCA reagent is added to every well containing standard curve or diluted sample.
After completion, the subsequent steps were conducted outside the FeliX:
- The plate is sealed by hand.
- Incubation of the plate for 30 minutes at 37 °C.
- Read absorbance at 562 nM on the EnSpire plate reader.
To generate the standard curve, known BSA concentrations (of the standards) are plotted against their corresponding absorbances. A linear regression is subsequently used to determine protein concentrations of cell lysate samples with an unknown protein content from their absorbances. An average of the two duplicated samples is then multiplied by the dilution factor used.
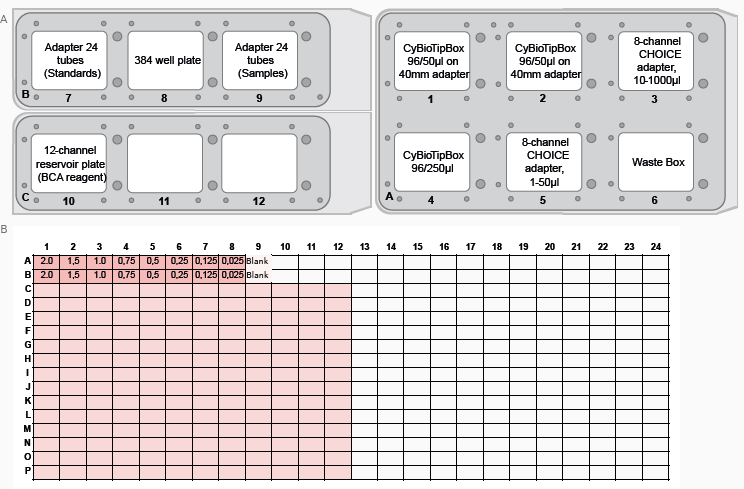
Figure 2. FeliX deck layout and plate well map for the BCA assay. (A) FeliX deck layout indicating the positions of tips, plates and tubes for preparation of the BCA assay. (B) Plate map for BCA assay (Standard curve – Orange, Blank – Yellow, Samples – Green). Image Credit: Analytik Jena US
Simple-Western Jess plate preparation (CyBio FeliX)
Providing the CyBio FeliX with the Jess plate template produced in the Simple-Western Compass software, and the protein concentrations produced in the BCA assay, means that the preparation of the Jess plate (shown in Figure 3) is nearly completely autonomous. The CyBio FeliX conducts the plate set-up as detailed in the steps below:
- Prepare the deck in adherence with the instructions presented by the CyBio FeliX. Figure 4 shows a layout of where to place items on the deck.
- Each cell lysate is diluted to the required concentration using a variable volume of 0.1x Sample buffer and a set volume of cell lysate in PCR tube strips.
- Small tubes of DTT and master mix are reconstituted using the proper volumes. 1.25 μL is then dispensed into fresh PCR tube strips.
- Move 5 μL of diluted cell lysate into the 1.25 μL of master mix.
- These PCR tube strips must be taken off FeliX and transported to a Thermocycler to be kept at 95 °C for 5 minutes. Steps 6 to 9 can be conducted while this occurs.
- The small tube of biotinylated ladder is reconstituted and 10 μL is transferred into well A1.
- 10 μL of Streptavidin-conjugate is transferred into well D1.
- The steps below are conducted by multi-dispense utilizing the 8-Channel CHOICE 10-1000μl adapter and a single 250 μL tip (ensure the tips are replaced for every reagent).
- 10 μL of Antibody dilution buffer is relocated into wells B1-B25 and C1.
- 10 μL of Primary antibody solution is relocated into the suitable wells between C2 and C25.
- 10 μL of Secondary antibody solution is relocated into the suitable wells between D2 and D25.
- If suitable, 15 μL of pre-mixed 1:1 Peroxide: Substrate is added to wells E1-E25.
- The PCR strips are returned to the cool block in the FeliX and 4 μL of the samples are transported into the analogous wells between A2 and A25
- 500 μL of wash buffer is added into all the wells of rows G, H, and I.
Before running it on the Simple-Western Jess, the plate is centrifuged at 1250 × g for 5 minutes, and alcohol vapor is added with a squeeze bottle — to ensure no bubbles, the internal straw is removed. The plate is then run on the Jess instrument utilizing the template presented to the CyBio FeliX at the start of set-up.
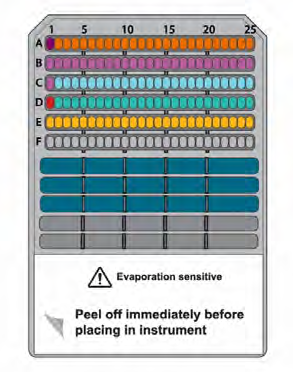
Figure 3. Schematic of the Simple Western Jess plate. Image Credit: Analytik Jena US
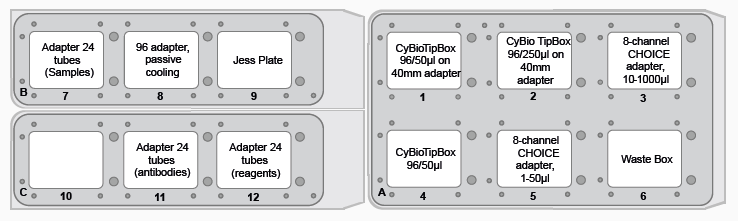
Figure 4. FeliX deck layout for Jess plate preparation. Image Credit: Analytik Jena US
Results and discussion
Antibody optimization (CyBio FeliX)
The Simple-Western Jess is sensitive and quantitative, so each assay must be optimized for its target. This optimization must also be specific to each cell line. To guarantee that the data received is truly quantifiable, concentration-dependent signal linearity and antibody saturation (two aspects of antibody optimization) must be accomplished.
To establish the activity of THAL-SNS-032, the anti-β-Actin, and the anti-CDK9 antibody were optimized using the CyBio FeliX. Utilizing an MV-4-11 lysate of known protein concentration, the CyBio FeliX utilized serially diluted lysate and varying antibody dilutions to prepare the Simple-Western Jess plate, eliminating the manual steps usually needed for antibody optimization.
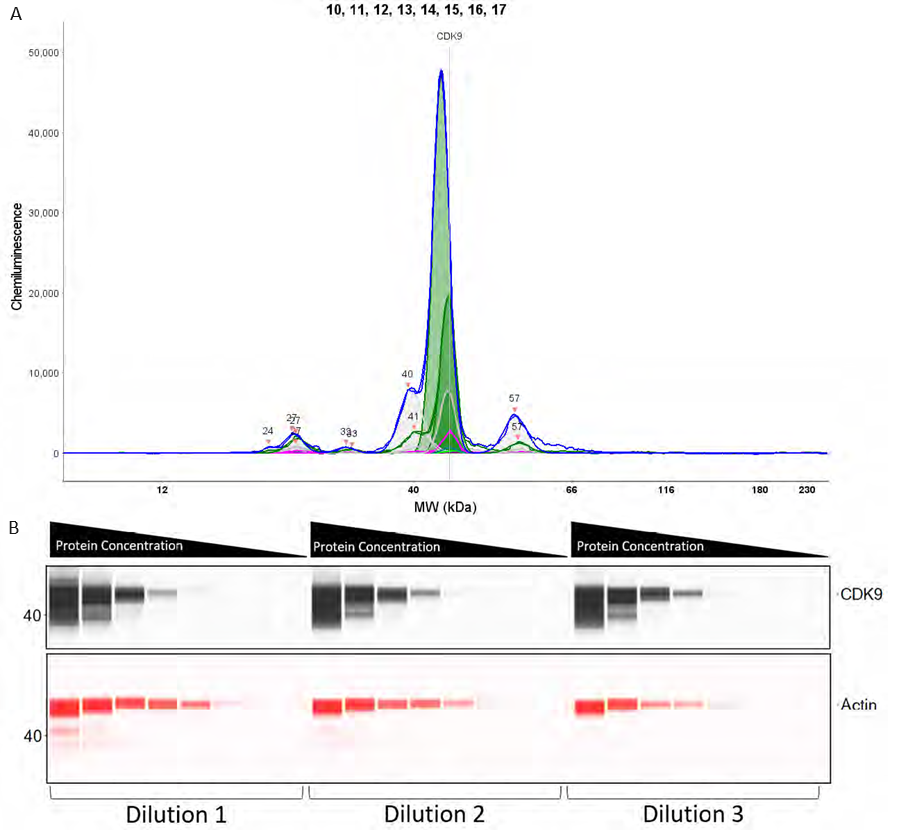
Figure 5. (A) Graph demonstrating the varying intensity of CDK9 at varying protein concentrations. (B) Pseudo-Image of CDK9 and β-Actin optimisation with 8 protein concentrations and 3 antibody dilution factors. Image Credit: Analytik Jena US
The luminescence or near-infrared (NIR) signal emitted from the glass capillary via the secondary antibodies is identified by the camera inside the Jess. It is converted into an intensity graph (Figure 5A). A pseudo-image representation of the graphs can be generated to give a conventional-style visualization of the data (Figure 5B). The peak height or area under the curve (AUC) is subsequently utilized to calculate the linearity and saturation of the antibody.
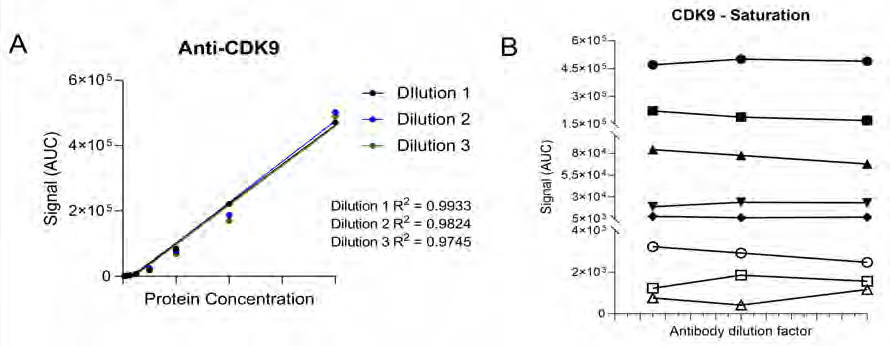
Figure 6. (A) Anti-CDK9 antibody demonstrates protein concentration-dependent signal linearity with R2 values >0.97 for all antibody dilution factors. This demonstrates the ability to detect differing target levels within samples. (B) A different symbol represents each protein concentration. At each protein concentration, the chemiluminescence signal is similar despite changing the antibody concentration, suggesting that the antibody is saturating the target protein in this cell line demonstrating that the antibody is well optimized under these conditions. Image Credit: Analytik Jena US
A graph that shows the AUC of the CDK9 signal on the Y-axis and the protein lysate concentration on the X-axis can subsequently be plotted. The R2 of the best-fit line represents the antibody's linearity (shown in Figure 6A). The linearity can be used to determine whether the antibody can identify variations in the target protein within the sample.
A graph plotting the AUC of CDK9 on the Y-axis and antibody dilution factor on the X-axis for each protein concentration can be used to virtualize the ability of the anti-CDK9 antibody to saturate its target (as shown in Figure 6B).
Antibody saturation assessed whether the epitopes of the target (CDK9 in this instance) are occupied, regardless of variations in antibody concentration. If fluctuations in signal are observed across the various antibody dilution factors, it indicates sub-optimal saturation (that is, not all the target epitopes are occupied).
CDK9 antibody optimization highlights that the signal remains identical across all antibody concentrations, suggesting that antibody concentration will not be a factor in the CDK9 signal in the following experiments.
The data indicates that the anti-CDK9 antibody is linear with an R2 >0.97 and saturated across all protein concentrations and antibody dilution factors (no antibody concentration-dependent changes).
This analysis was also conducted for the anti-β-Actin antibody in the NIR channel (which produced comparable results). This enabled the selection of a specific antibody dilution factor and protein concentration for every antibody.
Jess plate preparation (CyBio FeliX)
After optimization of the cell line protein concentrations and antibody dilution factors, cells are seeded and treated with serially diluted THAL-SNS-032 (500 nM top concentration with a 2/5 dilution factor). They are then washed with PBS and lysed with RIPA lysis buffer.
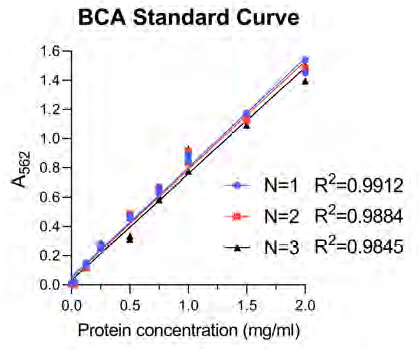
Figure 7. The BCA standard curve was constructed on each occasion to extrapolate unknown protein concentrations from the curve. On each occasion the technical reproducibility and linearity of the curve was performed with a high degree of accuracy. N=1, N=2, and N=3 reference biological replicates. Image Credit: Analytik Jena US
Following this, CyBio FeliX is employed to conduct the BCA protein assay, enhancing reliability and eliminating the manual requirements of assaying protein concentration for each of the 24 lysates created (Figure 7).
This step is critical, as it ensures that the cell lysates can be diluted to the required concentration—which was previously determined in the antibody optimization stages conducted by the CyBio FeliX. After the protein concentration of samples has been established, the Simple-Western Jess plate can be quickly and precisely prepared by CyBio FeliX.
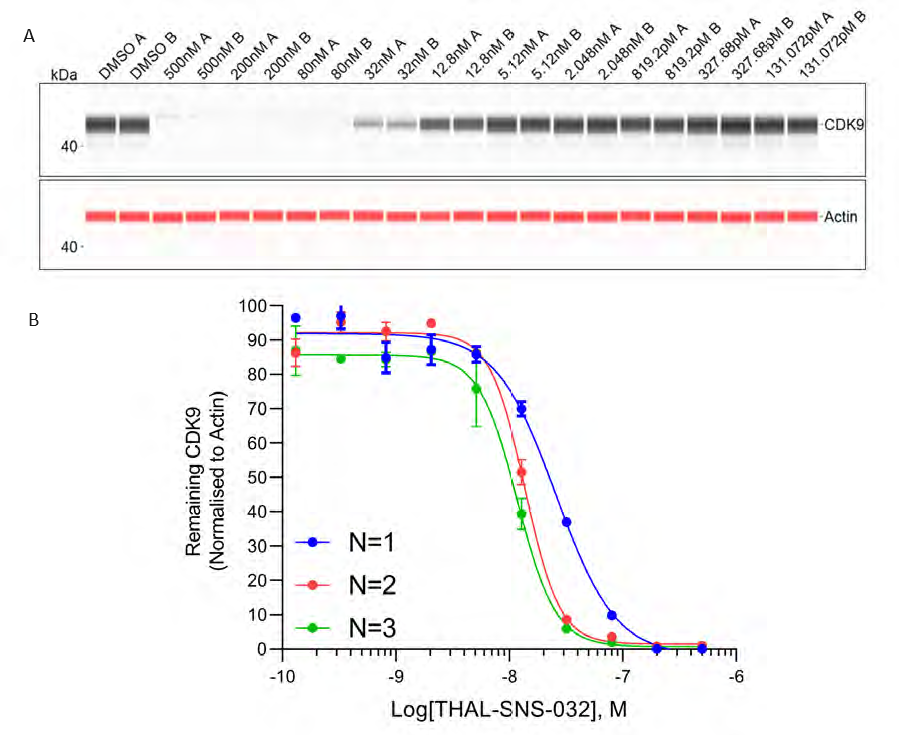
Figure 8. (A) Dose dependent depletion of CDK9 by THAL-SNS-032 visualised by the Jess pseudo-image for the first biological replicate (all concentrations run as duplicates A, B). (B) Dose-dependent depletion of CDK9 by SNS-THAL-032 for three individual biological repeats (N=1, N=2, N=3). Error bars represent standard deviation. Image Credit: Analytik Jena US
Three biological replicates were conducted to determine the reproducibility of the Jess plate preparation. The peak intensity data produced from each capillary is utilized to visualize the data (as shown in Figure 8A). These peaks can then be quantified and plotted as a dose-response curve (Figure 8B).
In all three replicates, SNS-THAL-032 demonstrated a reproducible and powerful dose-dependent degradation of CDK9 with a DC50 of 16.1 nM ± 7.6 nM. In all three biological replicates, consistent technical reproducibility is also apparent in each concentration, providing additional evidence of the precision and reliability of the CyBio FeliX.
Compared to a historical, manually prepared Jess, the Simple Western Jess plate preparation by the CyBio FeliX exhibited the ability to replicate data, reducing bench time by 60 minutes per Jess run (Figure 9).
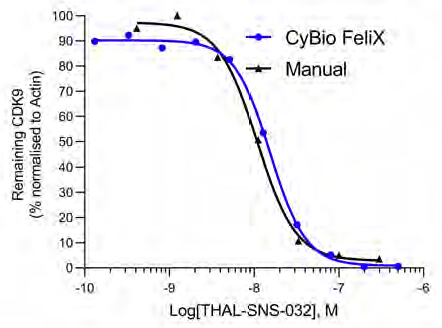
Figure 9. CDK9 depletion curves compare SNS-THAL-032 dose-response data from a hand-prepared Jess to the mean of the three biological replicates performed by the CyBio FeliX. Image Credit: Analytik Jena US
Conclusion
Combining the Simple-WesternTM Jess proficiency at Aurelia Bioscience with the liquid handling automation knowledge at Analytik Jena led to the development of a completely automated western blotting system. The CyBio FeliX processes cell lysates from the protein assay to quantifiable results, saving 60 minutes of bench time per Jess run.
The PROTAC THAL-SNS-032, a powerful degrader of CDK9, was used to demonstrate that the CyBio FeliX is a reliable and flexible liquid handling platform that can support the preparation of the Simple Western Jess plate and quantify variations in CDK9 concentrations within a sample.
It is crucial that these PROTAC assays can be used to quantifiably and reliably determine a target's level within a given sample. This stresses the importance of liquid handling accuracy, which the CyBio FeliX can deliver.
This platform can optimize the Simple Western Jess assay for novel targets by reliably optimizing sample protein and antibody concentrations. The manual approach improves flexibility and enables inexperienced researchers to use the technology.
About Analytik Jena US
Analytik Jena is a provider of instruments and products in the areas of analytical measuring technology and life science. Its portfolio includes the most modern analytical technology and complete systems for bioanalytical applications in the life science area.
Comprehensive laboratory software management and information systems (LIMS), service offerings, as well as device-specific consumables and disposables, such as reagents or plastic articles, complete the Group’s extensive range of products.
About life science
The Life Science product area demonstrates the biotechnological competence of Analytik Jena AG. We provide a wide product spectrum for automated total, as well as individual solutions for molecular diagnostics. Our products are focused to offer you a quality and the reproducibility of your laboratory results.
This will surely ease your daily work and speed up your work processes in a certain way. All together we support you through the complete process of the lab work. Besides we offer customized solutions and are able to adapt our products to your needs. Automated high-throughput screening systems for the pharmaceutical sector are also part of this segment’s extensive portfolio.
About analytical instrumentation
Analytik Jena has a long tradition in developing high-performance precision analytical systems which dates back to the inventions made by Ernst Abbe and Carl Zeiss. We have grown to become one of the most innovative manufacturers of analytical measuring technology worldwide.
Our business unit Analytical Instrumentation offers excellent competencies in the fields of optical spectroscopy, sum parameters and elemental analysis. Being proud of our core competency we grant all our customers a long-term warranty of 10 years for our high-performance optics.
About lab automation
With more than 25 years of market experience, Analytik Jena with its CyBio® Product Line is a leading provider for high quality liquid handling and automation technologies. In the pharmaceutical and life science industries, our products enjoy the highest reputation for precision, reliability, robustness and simplicity.
Moreover, the Automation Team designs, produces and installs fully automated systems tailored to our clients' application, throughput and capacity requirements. From stand-alone CyBio® Well up to fully customized robotic systems we handle your compounds, biomolecules and cells with great care.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.