In this article, Analytik Jena details what technology can make protein purification less variable and faster with the help of liquid-handling equipment.
The CyBio FeliX and PhyTips® can conduct 96 targeted protein purifications in 120 minutes, requiring no hands-on time.
These products are intended for drug discovery professionals in the biotechnology and pharmaceutical industry, bioprocess facilities, contract research organizations (CROs), academic institutions, start-ups, and government agencies.
Automated PhyTip® protein purification on the CyBio FeliX
PhyTip® columns from Biotage® are perfect for highly efficient protein purification on the CyBio FeliX liquid handler from Analytik Jena.
Every PhyTip® comprises an affinity resin bed that captures and keeps the protein bound during consecutive wash cycles. The purified protein is then released during elution. Various affinity resins are offered—they selectively immobilize tagged antibodies and proteins. Therefore, the combination of PhyTips® with the CyBio FeliX supplies a universal platform for purifying various proteins (including eukaryotic and bacterial cell lysates).
This article details the purification of histidine-tagged proteins utilizing immobilized metal affinity chromatography (IMAC) resin-filled PhyTip® columns. PhyTips® were combined with the CyBio FeliX—a small footprint 12-position liquid handler, 1-1000 μL in volume.
Bacterial lysates, containing recombinantly expressed histidine-tagged proteins and an extraction buffer, were loaded into the PhyTip® columns. Protein samples were washed and eluted into a collection plate after 120 minutes.
Protein concentration was evaluated using a Bradford assay. Purity was assessed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Mass spectrometry was then utilized to confirm the identity of the purified protein.
The CyBio FeliX and PhyTips® provide a rapid and convenient method to purify proteins at high purity and concentration. This method is completely walk-away and can be altered to target various protein features. The method can then be scaled to handle as many purifications as necessary.
Materials and methods
Labware and reagents
Bacterial growth and lysis
- BL21(DE3) Singles Competent Cells – Novagen, Sigma- Aldrich #70235-M
- Ampicillin – Merck® #A8351-25G
- Auto induction media – Formedium #AIMTB0210
- BugBuster® Master Mix – Merck® #71456
- Nunc 96 Well Polypropylene DeepWell Storage Plates – Thermo Fisher #278743
Protein purification
- 1000 μL PhyTip® columns, with 10 μL Ni-IMAC affinity resin - Biotage® #PTT-91-10-03
- 1000 μL PhyTip® columns, with 160 μL Ni-IMAC affinity resin - Biotage® #PTT-91-16-03
- 5x IMAC equilibration & capture buffer, 25 mM imidazole
- 5x IMAC wash Buffer: phosphate buffer, 25 mM imidazole
- 1x IMAC elution Buffer: 10 mM NaH2PO4, 0.3 M NaCl and 200 mM imidazole, pH7.4
- 5x Nunc 96-Well Polypropylene DeepWell Storage Plates – Thermo Fisher #278743
- 3x Axygen Single Well High Profile Reagent Reservoirs – Corning #RES-SW96-HP-SI
- 2x CyBio TipRack 96/1000 μL – Analytik Jena#OL3811-25-539-N
- 3x CyBio Support 97 mm – Analytik Jena #OL3317-11-105
- 3x CyBio RoboTipTray 1-96/1000 μL #OL3810-24-023
Protein quantification
- Bovine serum albumin (BSA) – New England Biolabs #B9000S
- Bradford Reagent – Merck® #B6916-500ML
- Greiner flat bottom 96-Well Plate, polystyrene – Greiner #65516
SDS gel electrophoresis
- Bolt 4-14 % Bis-Tris Plus Gel – Invitrogen #NW04120BOX
- Page Ruler Plus Prestained Protein Ladder – Thermo Fisher #26619
- InstantBlue® Coomassie Protein Stain – Abcam #ab119211
- 4X Bolt LDS Sample Buffer – Thermo Fisher #B0008
- 10X Bolt Sample Reducing Agent – Thermo Fisher B0009

Figure 1. 1000 μL PhyTip® columns on the CyBio FeliX. Between 1 to 96 PhyTips® can be used on the CyBio FeliX per purification cycle as required. Image Credit: Analytik Jena US
DNA purification
- E.Z.N.A.® Plasmid DNA Mini Kit II –Omega BIO-TEK
#D6945-002
- Isopropanol - Merck® #109634
- Ethanol - VWR® #20821.365
Protein digestion and identification
- Ammonium bicarbonate – Merck® #101131
- Dithiothreitol – Merck® #111474
- Iodoacetoamide – Merck® #804744
- Trypsin, porcine, sequencing grade, modified – Promega
Corp #9PIV511
- Water, HPLC grade – Merck® #V270733
- Acetonitrile, HPLC gradient grade – Merck® #100030
- Formic acid, HPLC – Merck® #543804
- Trifluoroacetic acid – Sigma #302031
Instruments
- CyBio FeliX Basic Unit with Enclosure – Analytik Jena #OL5015-24-100
- CyBio FeliX Head R 96/1000 μL – Analytik Jena #OL3316-14-950
- CLARIOstar® Plus microplate reader – BMG Labtech
- iBright FL1500 Imaging System– Thermo Fisher #A44241
Methods
Proteins, recombinantly expressed in BL21 (DE3) Escherichia coli strains, were chosen for purification using PhyTip® columns. The expression plasmid peT21a had a C-terminal 6 poly histidine-tag and a T7-Lacl promoter. The protein, tagged with histidine, is estimated to be 27.6 kDa in size.
Growth and lysis
Inoculations of expression cultures were formed using glycerol stocks in auto-induction media, supplemented with 100 μg/mL of ampicillin. Following a 16-hour incubation at 37 °C and 200 rpm shaking, bacteria were collected via 20-minute centrifugation at 2500 xg and 4 °C.
The supernatant was decanted, and 1 mL of lysis solution was introduced to the bacterial pellets. To resuspend them in lysis buffer, the pellets were placed on an orbital shaker for 30 minutes at 300 rpm. Lysed cells underwent another round of centrifugation at 21,000 xg for 20 minutes. Cleared lysate (1 mL) was transferred to a 96-deep well plate (#278743) ready for automated protein purification.
Plasmid isolation and sequencing
The plasmid sequence was established before PhyTip® purification. Following the manufacturers’ instructions, plasmid DNA was isolated from bacterial cultures with the Omega BIO-TEK E.Z.N.A.® Plasmid DNA Mini Kit II.
Ten microliters of plasmid DNA were subsequently submitted to Source BioScience for Sanger Sequencing. The plasmid was sequenced at 3.2 pmol/μL with the following primers; Forward TAA TAC GAC TCA CTA TAG GG, Reverse GCT AGT TAT TGC TCA GCG G.
Protein purification with the CyBio FeliX
The equilibration and wash buffers, supplied in the PhyTip® column kit at 5x concentration, were diluted to 1x concentration utilizing dH2O. Elution buffer was supplied at 1x concentration. The three buffers were poured into individual reservoirs (#RES-SW96-HP-SI). The required volume of each buffer is determined by the quantity of PhyTip® columns used in the automated run.
As highlighted in Figure 2, the pipetting tips and labware needed for the protein purification were placed onto the CyBio FeliX. After loading the CyBio FeliX deck, the purification program started, and the robot conducted the pipetting steps (described below).
- Buffer Transfer: Buffers supplied in single well reservoirs were aliquoted to 96 deep well plates utilizing 1000 μL tips.
- Equilibration: PhyTip® columns were equilibrated via numerous pipetting cycles of the equilibration buffer, priming the resin.
- Protein Capture: To bind the target proteins from the lysate to the resin, PhyTip® columns aspirated and dispensed the bacterial lysate numerous times.
- Wash: Through two separate plates, PhyTip® column resin was washed through aspirating and dispensing of wash buffer.
- Elution: Proteins attached to PhyTip® columns were released through multiple pipetting cycles in an elution buffer of 100 μL. The elution process was repeated in a second elution plate with 100 μL.
After the program was finished, purified proteins, eluted into two 96-well plates, were withdrawn from the instrument for further processing. The tips utilized to aliquot wash and equilibration buffer in step one can be used again in successive purifications.
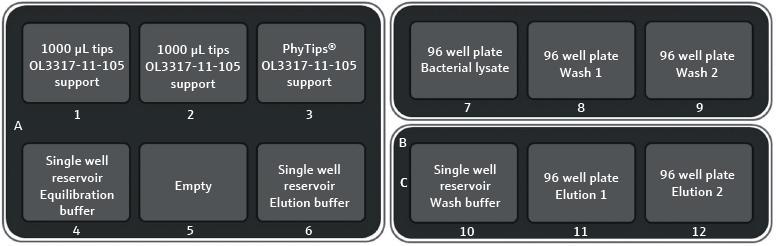
Figure 2. Deck layout of CyBio FeliX for PhyTip® protein purification. The operator places all labware and tips onto the CyBioFeliX deck at the start of the protocol, as instructed by the software. Plates on position 8, 9, 11 and 12 are placed onto the deck empty. They are then filled from their corresponding reservoirs during step one of the automated workflow before the purification section. Image Credit: Analytik Jena US
Protein quantification and purity assessment
A Bradford standard curve of bovine serum albumin (BSA) was utilized to establish protein concentration. Six concentrations of BSA protein standards were made at the following concentrations: 0.1, 0.2, 0.4, 0.6, 1.0, and 1.4 mg/mL.
5 μL of PhyTip® purified proteins and 5 μL of each protein standard were added to separate wells of a flat bottom 96 well plate. A blank well containing 5 μL elution buffer supplied in the PhyTip® kit was also added to the plate. Lastly, 250 μL of 1x Bradford reagent was added to every well. Following a 20-minute incubation in the dark at room temperature, absorbance was measured at 595 nm.
Alongside the starting bacterial lysate, proteins from the PhyTip® purification were additionally assessed using SDS-PAGE. 10 μL of sample buffer, 24.4 μL dH2O, and 4 μL of reducing agent were added to 1.6 μL of sample.
Samples were denatured for 10 minutes at 70 °C. 40 μL of samples were evaluated via SDS-PAGE, including 5 μL of ladder. Samples were run at at 200 V for 22 minutes at 200. Lastly, gels were stained in instant blue stain for 60 minutes and then de-stained in dH2O for 30 minutes. The iBright imaging system was employed to visualize the protein bands.
Protein Identification and purity assessment
Intact proteins in solution were initially characterized by liquid chromatography-mass spectrometry (LC/MS), utilizing an Agilent PLRP-S column and a gradient ranging from 20% to 50% acetonitrile, coupled with ESI-Q-ToF mass analysis. Spectra were obtained above the m/z range 300-3,200—protein mass was determined via deconvolution of the raw mass spectra.
Protein bands divided using SDS-PAGE were isolated and later submitted for proteomics evaluation to confirm that they correlated to the recipient protein—as detailed in Shevchenko et al.1
The most prominent protein bands—corresponding to molecular weights of 10, 25, and 30 kDa in purified samples—were removed and centrifuged. The gel slices were incubated with acetonitrile and ammonium bicarbonate buffer to remove the protein stain. They were then saturated with trypsin to produce peptides.
These peptides were detected by LC/MS/MS utilizing an ESI-Q-ToF set in auto-MS/MS mode. Peptides were removed from an Agilent AdvanceBio Peptide column with a 2%-40% acetonitrile gradient over 12 and a half minutes.
The accumulated spectra were searched against custom sequences or a SwissProt database using SpectrumMill (Agilent).
Results and discussion
Protein yield
The histidine-tagged proteins were approximately 27.6 kDa in size. They were purified with PhyTip® columns filled with 10 or 160 μL of purification resin. A Bradford assay was used to assess protein concentration (Figure 3).
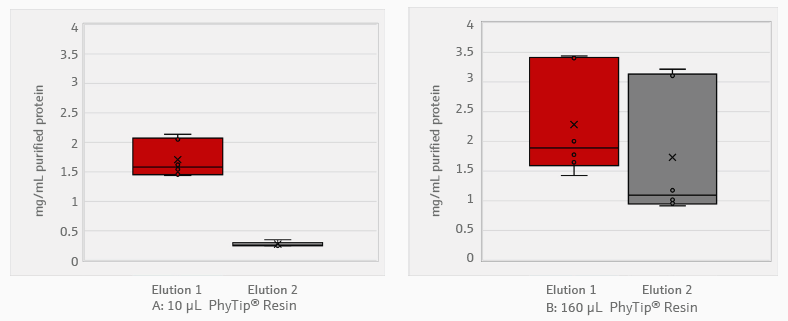
Figure 3. Concentration of proteins purified with PhyTip® resin. A total of 1000 μL of bacterial expression culture grown for 16 hours was used for protein purification. The histidine-tagged proteins were captured via 10 μL (A) and 160 μL (B) PhyTip® resin columns. Proteins were eluted in two separate, successive elution steps of 100 μL of elution buffer. Protein concentration was measured using Bradford assay. All steps from bacterial growth to purification were performed in triplicate. Data is represented as a box plot, where the central line represents the median, and the X represents the mean. N = 3. Image Credit: Analytik Jena US
Protein yield was correlated to the total volume of PhyTip® resin. The 10 μL resin tips yielded an average 1.0 mg/mL purification in 200 μL of elution buffer. Most of the protein, 1.7 mg/mL, was removed in the first elution stage: the second elution only contained 0.3 mg/mL of protein.
The 160 μL tips yielded an average purification of 2.0 mg/mL in 200 μL of elution buffer. With these tips, the concentration of the second elution was 1.7 mg/mL—76% of the initial elution. Further elution steps or larger elution volumes can recover additional protein.
The purified proteins, removed with the 10 μL resin tips in the first and second stages, were loaded onto SDS-PAGE (Figure 4). Three prominent bands were observed around 10, 25, and 30 kDa. Mass spectrometry analysis revealed that the bands at 25 and 30 kDa correlated to the protein sequence of the target. The band at 10 kDa correlated to an E. coli outer membrane lipoprotein, also present in the bacterial lysate.
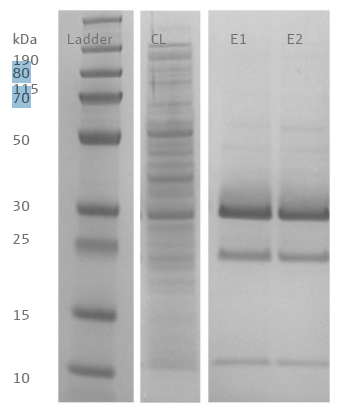
Figure 4. SDS-PAGE of proteins purified with PhyTip® resin columns. Lane 1: Ladder, Lane 2: Cleared bacterial lysate, Lane 3: Elution step one, Lane 4: Elution step two. Peptides from the targeted histidine-tagged protein was identified in the bands migrating to a height equivalent to 25 and 30 kDa. Image Credit: Analytik Jena US
Discussion
The use of PhyTip® columns on the CyBio FeliX permits rapid, high-throughput protein purification. The final concentration and yield of the recovered protein are associated with the quantity of PhyTip® resin used for the purification.
SDS-PAGE was employed to evaluate protein purification. The most intense protein band, isolated with PhyTip® columns, correlated to the predicted weight of 28 kDa. A possible truncated isoform was additionally detected—this is also present in the bacterial lysate. Introducing protease inhibitors to the lysis buffer could minimize proteolysis, reducing secondary bands. The additional bands are most likely endogenous E. coli proteins with native histidine residue clusters, often observed in purification approaches that target histidine-tagged proteins.2
Summary
High-throughput protein purification is technically difficult and time-consuming. Using PhyTip® columns can purify protein targets with high yields and purity in a few easy steps, with minimal assay optimization needed. Together, the CyBio FeliX and automated PhyTip® columns enable the processing of 96 protein purifications in less than two hours.
The workflow is entirely automated, enhancing reproducibility between sample extractions while liberating researchers’ time for additional applications.
Automation of PhyTip® columns with the CyBio FeliX enables high-throughput protein purification in a compact and powerful format. Pre-written methods are obtainable for the CyBio FeliX to perform PhyTip® purifications in a flexible and accessible format.

Figure 5. CyBio FeliX. Image Credit: Analytik Jena US
References and further reading
- Shevchenko, A., Tomas, H., Havli, J. et al. “In-gel digestion for mass spectrometric characterization of proteins and proteomes”. Nat Protocols 1, 2856–2860 (2006). doi:10.1038/nprot.2006.468.
- Robichon C, Luo J, Causey TB, Benner JS, Samuelson JC. Engineering „Escherichia coli BL21(DE3) derivative strains to minimize E. coli protein contamination after purification by immobilized metal affinity chromatography.” Applied and environmental microbiology, 77,13 4634-46. (2011). doi:10.1128/AEM.00119-11.
About Analytik Jena US
Analytik Jena is a provider of instruments and products in the areas of analytical measuring technology and life science. Its portfolio includes the most modern analytical technology and complete systems for bioanalytical applications in the life science area.
Comprehensive laboratory software management and information systems (LIMS), service offerings, as well as device-specific consumables and disposables, such as reagents or plastic articles, complete the Group’s extensive range of products.
About life science
The Life Science product area demonstrates the biotechnological competence of Analytik Jena AG. We provide a wide product spectrum for automated total, as well as individual solutions for molecular diagnostics. Our products are focused to offer you a quality and the reproducibility of your laboratory results.
This will surely ease your daily work and speed up your work processes in a certain way. All together we support you through the complete process of the lab work. Besides we offer customized solutions and are able to adapt our products to your needs. Automated high-throughput screening systems for the pharmaceutical sector are also part of this segment’s extensive portfolio.
About analytical instrumentation
Analytik Jena has a long tradition in developing high-performance precision analytical systems which dates back to the inventions made by Ernst Abbe and Carl Zeiss. We have grown to become one of the most innovative manufacturers of analytical measuring technology worldwide.
Our business unit Analytical Instrumentation offers excellent competencies in the fields of optical spectroscopy, sum parameters and elemental analysis. Being proud of our core competency we grant all our customers a long-term warranty of 10 years for our high-performance optics.
About lab automation
With more than 25 years of market experience, Analytik Jena with its CyBio® Product Line is a leading provider for high quality liquid handling and automation technologies. In the pharmaceutical and life science industries, our products enjoy the highest reputation for precision, reliability, robustness and simplicity.
Moreover, the Automation Team designs, produces and installs fully automated systems tailored to our clients' application, throughput and capacity requirements. From stand-alone CyBio® Well up to fully customized robotic systems we handle your compounds, biomolecules and cells with great care.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.