Chronic damage or injury to nerves causes changes in the primary sensory neurons located in the dorsal root ganglion (DRG) and in their central connections, as shown in Figure 1. Activation of galanin receptors (GalR1, GalR2 and GalR3) mediates the physiological effects of galanin. These receptors inhibit adenylyl cyclase. GalR2 also mediates calcium mobilization at the intracellular level. The galanin system, especially GalR2, is implicated in the regulation of nociception, partly based on a significant 120-fold up-regulation in the galanin levels observed in the DRG following nerve injury.
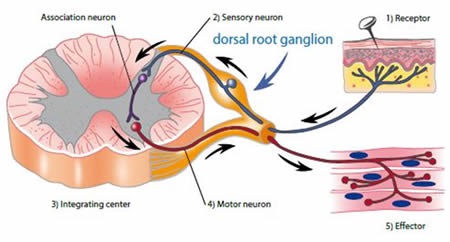
Figure 1. Nociceptive pathways. Image credits: BMG Labtech.
Potentiation of peripheral GalR2 activity induced by galanin should eventually cause a marked reduction in nociceptive responses and provide innovative treatments for neuropathic pain. Unlike direct orthosteric agonist activation, positive allosteric modulators (PAMs) of GalR2 could afford further therapeutic benefits. These benefits include retention of physiologically-controlled temporal and spatial resolution, enhanced receptor-subtype selectivity, a self-restricting saturability of effect and utilization of untapped chemical space. Confirmed and selected PAMs could offer new treatments and IP solutions for various clinical requirements that are yet unmet. In this analysis, a high-throughput screen of GalR2 was used to identify new therapeutics for neuropathic pain. This screen used the MRCT 100K compound collection, which is a selection of drug-like molecules obtained from commercial libraries, comprising more than 10,000 compounds that target interactions between proteins.
HTRF® Technology
Cisbio is a French-based company that invented and further develops the HTRF® technology to create reagents, kits and services for drug discovery research. The company’s homogenous assays are used in life science to study biomarkers, G-protein-coupled receptors (GPCRs), epigenetics, and kinases and signaling.
The multidetection microplate readers CLARIOstar, PHERAstar FS, FLUOstar Omega, and POLARstar Omega are all HTRF-certified by Cisbio, meaning each of the devices fulfill Cisbio's expectations with respect to dynamic range, signal-to-noise ratio, sensitivity, CV%, and DF%.
PHERAstar FS features
The PHERAstar FS is an advanced HTS plate reader designed for all HTRF assays and features the following: 
- All microplate formats up to 3456-well
- Dedicated UV-laser excitation
- HTRF-specific evaluation templates
- HTRF-dedicated optic modules
- Three integrated bar code readers
- Two matched, HTRF-dedicated PMTs
- Fast read times with simultaneous dual emission
The CLARIOstar features
Offering the following features, the CLARIOstar is one of the most versatile plate readers available on the market:
- High quality HTRF measurements
- HTRF-dedicated filters
- High energy xenon flash lamp
- HTRF-specific evaluation templates
Principle of the Assay
Cisbio’s robust HTRF® functional IP1 assay is based on a competitive immunoassay principle, whereby free IP1 competes with the HTRF® acceptor IP1-d2 in binding to the HTRF® donor, anti-IP1 Cryptate conjugate. The signal is inversely proportional to levels of IP1 in the cell with maximum FRET obtained when IP1 is absent, as shown in Figure 2. The HTRF® functional IP1 assay was used for screening.
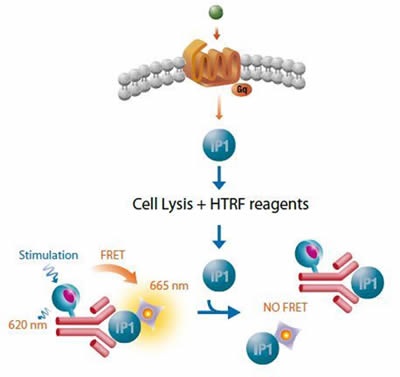
Figure 2. Cisbo IP-One HTRF assay. Image credits: BMG Labtech.
Materials and Methods
The following materials were used for the analysis:
- Cisbio’s IP-One HTRF® assay kit
- GE Healthcare’s CHO cells stably expressing GalR2
- BMG LABTECH’s PHERAstar microplate reader with HTRF optic module [620/665]
- Greiner’s white 384-well small volume plates
CHO cells stably expressing GalR2 were used to configure the assay. Cells were pre-incubated using a sub-maximal concentration of the galanin agonist, sensitizing the HTS assay to the simultaneous detection of PAMs and agonists. The MRCT 100K compound collection was screened at a 10µM final assay concentration.
5µl of cells per well were dispensed and 2.5µl buffer control or compound was added to 384-well low volume white plates. After an incubation period of 30 min at a temperature of 37°C, 2.5µl of galanin was introduced at an above-minimal concentration of 3.16nM (EC20) or a maximal concentration of 1mM (EC100). Buffer comprising 0.1% bovine serum albumin (BSA) was added to the test samples and plates were incubated for a period of 1 hr at a temperature of 37°C. Next, each well received 5µl of each HTRF reagent and a 1 hr incubation was performed at room temperature. The PHERAstar microplate reader was then used to read the plates.
Settings for the PHERAstar Instrument
The HTRF optic module was used to carry out the readings. This module excites at 337nm wavelength and detects emission at 665/620nm simultaneously. Using a focal height of 12.3mm, integration was carried out for 400µs, beginning at 50µs using 400 flashes for each well. A ratio multiplier of 10,000 was used.
Data Calculation
Normalization of data was carried out by calculating the ratio of the raw data acquired at 620 and 665nm as follows: Ratio = (665/620)*10000. This was expressed relative to EC20 and EC100 such that EC100 = 100% and EC20 = 0%, as illustrated in Figure 3. A 30% response cut off or 30% above EC20 was used to select hits.
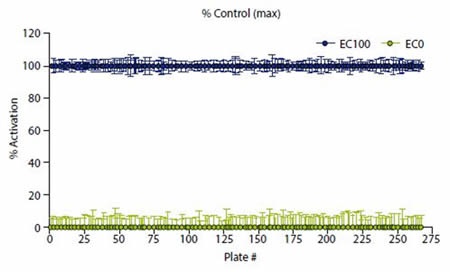
Figure 3. Data were normalized to high (EC100) and low ( EC20) controls. Image credits: BMG Labtech.
Results and Discussion
As indicated by the consistent and robust Z’ data (Figure 4), the HTS assay performed well. A mean Z’ of 0.72 (± 0.05) was obtained for 85320 compounds screened. In addition, the %CV for the control wells was low – High Control %CV = 5.0 (±0.8) and Low Control %CV = 3.7 (± 1.9). The number of hits depending on cutoff is shown in Table 1. 250 compounds are selected for validation analyses.
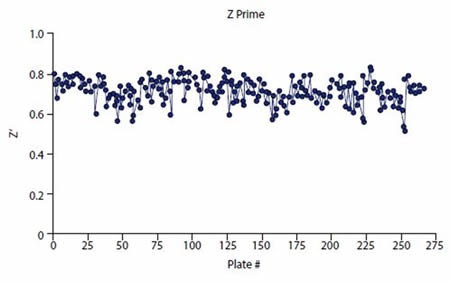
Figure 4. Z prime values consistently exceeded plate pass/fail acceptance criteria (Z’ > 0.5). Image credits: BMG Labtech.
Table 1: Number of hits attained during screening
| Cutoff (%) |
#Hits
|
|
30
|
250
|
|
40
|
117
|
|
50
|
65
|
|
60
|
29
|
|
70
|
13
|
|
80
|
9
|
|
490
|
4
|
|
100
|
1
|
Conclusion
The screen provided a fairly low hit rate (0.3% at 30% activity), with the majority confirmed in PAM mode. Most of the compounds, however, were also found to be active agonists. More studies are being conducted against an extra 80 K library. Since the above results validate this application, the same approach can be employed with the PHERAstar (Figure 5).

Figure 5. BMG LABTECH’s multidetection microplate reader PHERAstar. Image credits: BMG Labtech.
Acknowledgement
Produced from articles authored by Hayley Jones, Jeff Jerman, MRC Technology, London; David Wynick, University of Bristol, Bristol, UK; Catherine Wark, Carl Peters, Ph.D., BMG LABTECH, Cary, NC.
About BMG Labtech

BMG LABTECH has been committed to producing microplate readers for more than twenty years. By focusing on the needs of the scientific community, the company’s innovative microplate readers have earned the company the reputation of being a technology leader in the field.
BMG LABTECH has developed a wide range of dedicated and multi-mode microplate readers for life sciences applications and high-throughput screening.
All BMG LABTECH microplate readers are "Made in Germany" and are conceived, developed, assembled, and tested entirely at our headquarters in Germany.
Since our establishment in Offenburg, Germany in 1989, BMG LABTECH has expanded to offer a worldwide sales and support network with offices in the USA, UK, Australia, Japan and France. Our subsidiaries, regional offices and distributors are committed to bringing you innovative microplate reader technology with the quality and reliability you expect from a German company.
Our staff includes engineers and scientists from the fields of biology, biochemistry, analytical chemistry, and physics.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.