To understand biochemical reactions, measurement of stoichiometry is crucial. This is because these reactions depend on the interaction of at least two cellular components. Measuring these interactions has been limited to methods which are useful for examining large molecular weight changes, like size-exclusion chromatography.
A large volume of purified cellular components is necessary for utilizing these methods. By considering these limitations a platform where protein-protein interactions could be observed could be provided.
Job plot1 is described below. The technique from BMG Labtech utilizes a microplate reader, which was employed to read FRET and relevant acceptor and donor control intensities.
The use of small sample volumes (40 μl) in 384-well plates is enabled by utilizing a microplate reader, four separate interactions may be examined on an individual plate.
Assay Principle
One key part of the Job plot, also known as the continuous variation method, is that the total concentration of the two molecules is kept constant.2 It is the ratio of the two molecules which is altered.
An observable parameter to complex formation is used, FRET between the labeled proteins is used in this instance. The expected assay results for a 1:1 protein-protein interaction are shown in Figure 1.
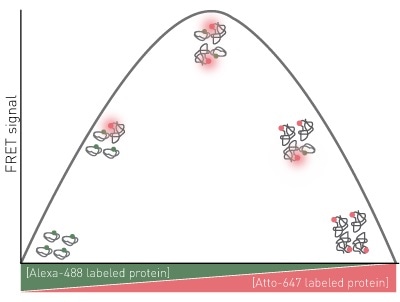
Figure 1. Assay Principle for FRET Job plot
There is no FRET signal in conditions where 100% donor labeled protein are present because the ratio shifts to larger amounts of acceptor, the FRET signal increases until maximum FRET is attained.
The stoichiometry of the interaction is indicated by maximum FRET. Continued modifications in the ratio toward greater acceptor will be associated with a decrease in FRET until zero FRET is observed again with 100% acceptor labeled protein.
Methods and Materials
- BMG Labtech CLARIOstar
- Black 384-well microplates (Corning)
- For a complete list of reagents and procedure please see Mattiroli et al.1
Instrument Settings
Due to the multichromatic nature of the test carried out, appropriate setting of the gain for each chromatic was key. In order to measure the acceptor, the fluorescence gain was set by utilizing a sample with highest acceptor dye and no donor, for example well P3.
Similarly, gain for donor fluorescence measurement was set on no acceptor dye and well with highest donor dye, for example well D1. For FRET measurement the plate is first read and the well with max FRET signal is identified. This well is employed to carry out gain adjustment.
Experimental Procedure
Stock solutions that possessed a 1 μM concentration were prepared for each protein, both unlabeled and labeled. Further dilution of these stocks was created so that the diluted stocks had a 2X concentration required for the well reactions.
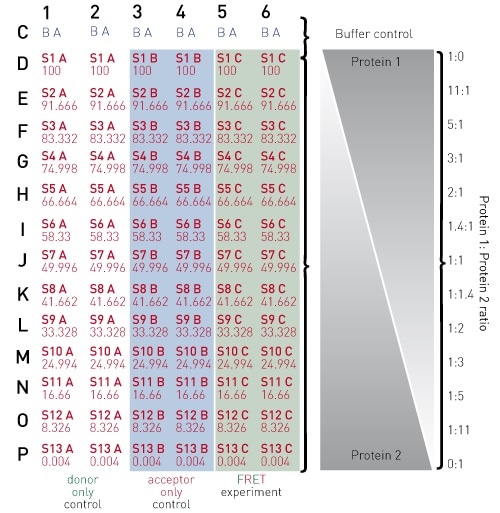
Figure 2. 384-well plate preparation layout
By combining 20 µl each of the appropriate diluted stocks for proteins 1 and 2, final well reactions are created. This can be performed according to the scheme depicted in Figure 2.
| . |
. |
. |
| Optic settings |
Fluorescence, multichromatic, endpoint |
| Chromatic 1: Alexa 488 preset |
| LVF Ex |
488-14 |
| Dichroic |
Auto: 507.5 |
| LVF Em |
535-30 |
| Chromatic 2: Atto 647 preset |
| LVF Ex |
625-30 |
| Dichroic |
Auto: 647.5 |
| LVF Em |
680-40 |
| Chromatic 3: Alexa 488/Atto 647 FRET |
| LVF Ex |
488-15 |
| Dichroic |
Auto: 577.8 |
| LVF Em |
680-40 |
| Gain |
Adjusted as described |
| General settings |
Number of flashes |
50 |
| Settling time |
0.1 s |
Results and Discussion
To begin with, as a proof of principle, the interaction between histone binding protein and histone H3-H4 was observed. In the layout described in Figure 2, histone binding protein is protein 1 and H3-H4 is protein 2. The expected 1:1 binding interaction is shown in Figure 3.
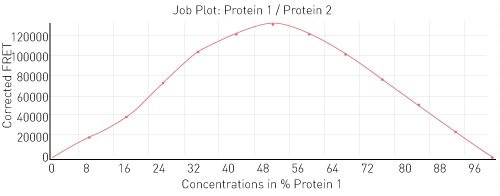
Figure 3. Job plot for interaction between H3-H4 and histone binding protein
The results of two additional protein interaction tests can be seen in Figure 4. As shown, one of the interactions also demonstrates a 1:1 stoichiometry while the other is an example of a 2:1 interaction stoichiometry.
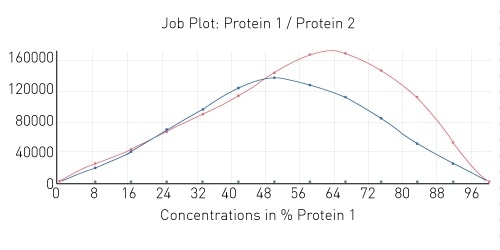
Figure 4. Job plot depicting 1:1 and 2:1 protein-protein interaction stoichiometry
Conclusion
The Job plot to examine protein-protein interaction stoichiometry has been adapted successfully to a microplate reader-based system. This allows both miniaturization to save on protein components and enhanced throughput, up to four protein pairs can be assessed in one 384-well plate.
References
- Mattiroli, F. et al. FRET-based Stoichiometry Measurements of Protein Complexes in vitro. Bio. Protoc. (2018) 8: e2713. DOI: 10.21769/BioProtoc.2713
- Huang, C.Y. Determination of Binding Stoichiometry by the Continuous Variation Method: The Job Plot. Methods Enzymol. (1982) 87: 509-525
About BMG Labtech

BMG LABTECH has been committed to producing microplate readers for more than twenty years. By focusing on the needs of the scientific community, the company’s innovative microplate readers have earned the company the reputation of being a technology leader in the field.
BMG LABTECH has developed a wide range of dedicated and multi-mode microplate readers for life sciences applications and high-throughput screening.
All BMG LABTECH microplate readers are "Made in Germany" and are conceived, developed, assembled, and tested entirely at our headquarters in Germany.
Since our establishment in Offenburg, Germany in 1989, BMG LABTECH has expanded to offer a worldwide sales and support network with offices in the USA, UK, Australia, Japan and France. Our subsidiaries, regional offices and distributors are committed to bringing you innovative microplate reader technology with the quality and reliability you expect from a German company.
Our staff includes engineers and scientists from the fields of biology, biochemistry, analytical chemistry, and physics.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.