Disruption of normal metabolism due to poor insulin secretion or resistance to insulin leads to diabetes mellitus1. In the year 2014, it was reported that diabetes is present in almost 10% of the U.S. population with type-2 diabetes accounting for 90% of diabetes cases2,3.
Diabetes continues to be a key focus to find new therapeutic interventions, as this disease is highly prevalent and requires large amounts of money for treatment.
As shown in Figure 1, physiologically active insulin secretion is regulated at various steps, each step representing a possibility for therapeutic intervention4.
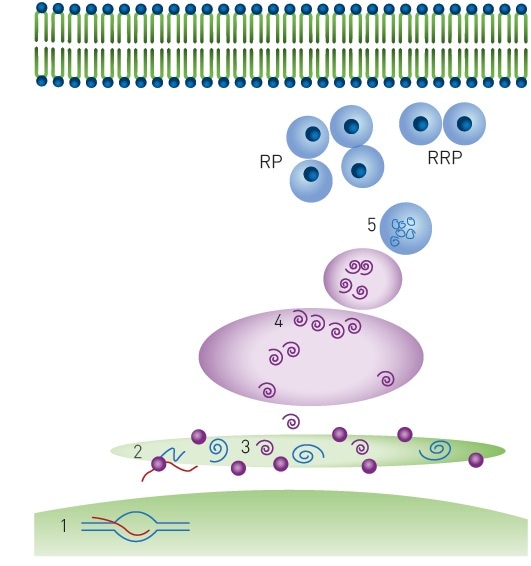
Figure 1. Insulin production and storage. Key biological steps in insulin production: 1) Transcription, 2) Translation and translocation to the endoplasmic reticulum, 3) Folding and signal peptide cleavage 4) Golgi transport and packaging into secretory vesicles 5) Cleavage to produce mature insulin. Mature insulin is stored in dense-core granules in two populations: RRP = ready releasable pool and RP = reserve pool. Image credits: BMG Labtech.
Assay principle
This article demonstrates a high throughput compatible cell-based assay that employs a preproinsulin-mCherry (PPI-mCherry) system. The study takes advantage of the fact that FRET can take place between the same types of fluorophores if a fluorophore is at a high local concentration.
This phenomenon is known as homoFRET (HF). In addition, if polarized light is used for excitation, it becomes randomized when HF occurs between neighboring fluorophores. It was believed that an HF-FP approach is appropriate to track the level of storing mature insulin into dense core granules, as shown in Figure 2.
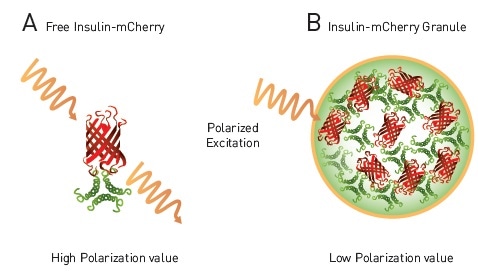
Figure 2. homoFRET-FP to detect packaging of insulin in dense core granules in live cells. A) Free insulin-mCherry with polarized excitation will exhibit conserved polarization and relatively high MP signal. B) Within dense core granule polarized light will exhibit homoFRET, randomized polarization and a decrease in MP signal4. Image credits: BMG Labtech.
Materials and methods
The following materials and methods were used:
- Preproinsulin (PPI) mCherry Reporter Construct was developed at UNC’s Dr. Brenman’s lab
- Rat insulinoma (INS-1) cells were donated by Christopher Newgard at Duke University
- 384-well black/clear bottom plates (NUNC #152029)
- 502 purified natural products (Enzo Life Sciences)
- 1,280 molecule FDA-approved drug set (Prestwick Chemical Library)
- PHERAstar® microplate reader from BMG LABTECH
After the INS-1 cells were transfected with PPI-mCherry, they were grown for a period of 48 hours and subjected to the specified concentrations of antagonists/agonists for 4 hours.
PHERAstar instrument settings
|
Measurement type
|
Fluorescence polarization
|
|
Measurement mode
|
End point
|
|
Optic module
|
FP(590-50/675-50/675-50)
|
|
Gain
|
Adjusted prior to test run
|
|
Target mP value
|
400
|
|
Focal height
|
7.2
|
|
Flashes / well
|
200
|
Results and discussion
In order to verify the HF-FP approach, the cells were treated with Bafilomycin, a vacuolar-type H+ ATPase (V-ATPase) inhibitor that blocks the maturation of vacuoles, and consequently blocks the formation of insulin granules (Figure 3). The results demonstrate that growing concentrations of Bafilomycin lead to increased mP values that correlate with reduced formation of granules.
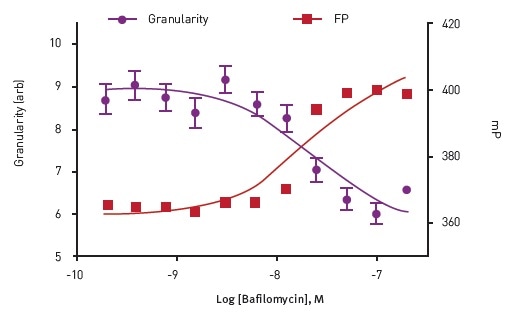
Figure 3. Dose response to Bafilomycin in Insulin Granule Packing Assay. Analysis show an anti-correlation between homoFRETFP signal and mCherry granularity. Image credits: BMG Labtech.
For successive experiments, 83 nM Bafilomycin was used as a positive control in comparison with DMSO negative control. Two different compound libraries were used to perform pilot screening.
The results obtained from these experiments revealed a Z’-factor, indicating that the assay is suitable for HTS. In addition, 26 compounds were also found to be active, as illustrated in Figure 4.
![FP data from pilot screen. Scatter plot of 1,782 test compounds (green) [hits (green stars)], negative control (DMSO - red) and positive control (Bafilomycin - blue). Arrows indicate Antimycin A1 is selected from both libraries.](https://www.news-medical.net/image-handler/picture/2017/3/BMG4.4.jpg)
Figure 4. FP data from pilot screen. Scatter plot of 1,782 test compounds (green) [hits (green stars)], negative control (DMSO - red) and positive control (Bafilomycin - blue). Arrows indicate Antimycin A1 is selected from both libraries. Image credits: BMG Labtech.
The representative confirmation of the three hits from the pilot screen is depicted in Figure 5. On the whole, the pilot screen showed a hit rate and a confirmation rate of 1.4% and 36.4%, respectively.
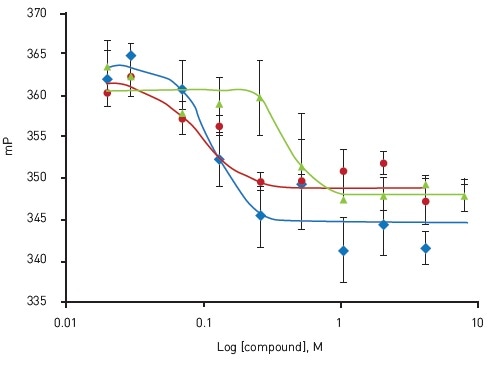
Figure 5. Dose-response confirmation of active compounds. Oligomycin A (blue); EC50 = 0.114 μM, Antimycin A1 (red); EC50 = 0.089 μM, Rotenone (green); EC50 = 0.37 μM. Adapted from Yi et al.4 Image credits: BMG Labtech.
Conclusion
The above results show that a new cell-based FP biosensor is capable of detecting compounds that alter the packaging of insulin granules. This technology could be used for assessing the interactions of proteins in live cell systems.
Acknowledgements
Produced from materials originally authored by N.Y. Yi1, Q. He1, T.B. Caligan1, G.R. Smith1, L.J. Forsberg2, J.E. Brenman2 and J. Z. Sexton1
1 North Carolina Central University, Durham, NC
2 UNC Chapel Hill School of Medicine, Chapel Hill, NC
References
- Rorsman P et al. (2000) The Cell Physiology of Biphasic Insulin Secretion. News Physiol. Sci. 15:72-77.
- Centers for Disease Control and Prevention (CDC) (2014) National Diabetes Statistics Report.
- Huang CJ et al. (2007) High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated beta-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes 56(8): 2016-2027.
- Yi NY et al. (2015) Development of a Cell-Based Fluorescence Polarization Biosensor Using Preproinsulin to Identify Compounds That Alter Insulin Granule Dynamics. Assay Drug. Dev. Technol. 13(9):558-569.
About BMG Labtech

BMG LABTECH has been committed to producing microplate readers for more than twenty years. By focusing on the needs of the scientific community, the company’s innovative microplate readers have earned the company the reputation of being a technology leader in the field.
BMG LABTECH has developed a wide range of dedicated and multi-mode microplate readers for life sciences applications and high-throughput screening.
All BMG LABTECH microplate readers are "Made in Germany" and are conceived, developed, assembled, and tested entirely at our headquarters in Germany.
Since our establishment in Offenburg, Germany in 1989, BMG LABTECH has expanded to offer a worldwide sales and support network with offices in the USA, UK, Australia, Japan and France. Our subsidiaries, regional offices and distributors are committed to bringing you innovative microplate reader technology with the quality and reliability you expect from a German company.
Our staff includes engineers and scientists from the fields of biology, biochemistry, analytical chemistry, and physics.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.