Screening facilities need to take necessary measures continuously for increased throughput, reduced sample expenditure, better control over cost, and eventually optimized efficiency. However, compromising data quality for saving samples, time and money is meaningless.
This article demonstrates the performance of the PHERAstar® FSX plate reader to detect a multiplex Alpha Technology (AlphaLISA® –AlphaPlexTM) type assay with two emission fluorophores. The concurrent dual emission detection of this assay on the PHERAstar FSX enables users to obtain double the information from each well at the same time taken by a standard AlphaLISA plate.
Assay Principle
The AlphaLISA principle is well defined. AlphaPlex, a modified AlphaLISA, that has been recently launched, has an emission wavelength with a peak 545nm rather than the conventional 615nm. As a result, changes in amount of two different targets can now be monitored simultaneously in the same well (Figure 1). The AlphaLISA SDE optic module allows simultaneous measurement of both emission wavelengths.
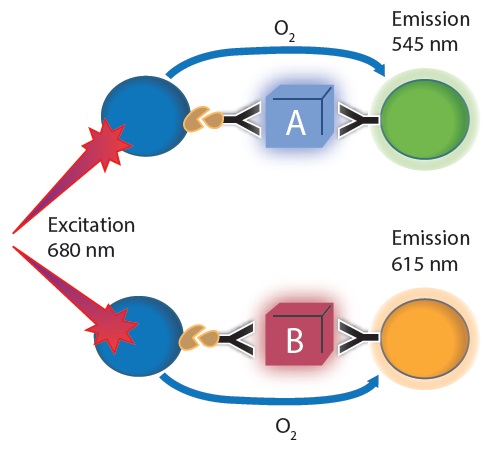
Figure 1. Multiplex Alpha technology principle: AlphaLISA and AlphaPlex used at once. For each protein target one antibody was conjugated to an acceptor bead and a 2nd biotinylated antibody was used. Streptavidin coated donor beads were added subsequently. Image credit: BMG Labtech.
Materials and Methods
- PHERAstar FSX (Figure 2)
- Packard proxiplate F, 384 well, black
- Purified proteins (Target 1 & 2), Antibodies
- AlphaLISA Acceptor beads
- AlphaPLEX 545 Acceptor beads
- Streptavidin Alpha donor beads

Figure 2. The PHERAstar FSX. The new gold standard for HTS featuring simultaneous dual emission detection. Image credit: BMG Labtech.
For the two protein targets, antibody pairs were chosen individually. The next step was conjugating one of the paired antibodies for each protein target to either AlphaLISA acceptor beads or AlphaPLEX 545nm acceptor beads (for protein target 1) and biotinylating the other antibody of each pair.
Preparation of two fold serial dilution of protein target 1 from an initial concentration of 256μg/mL was then carried out for test purposes. The varying concentrations were mixed with either 128μg/mL of target protein 2 or buffer for preparing plates. This was followed by preparing and plating a similar dilution series of target protein 2 with either 128μg/mL of target protein 1 or buffer.
The next step was preparing and adding a mixture consisting of biotinylated antibodies and acceptor beads for both target proteins to the reaction plates. After incubating for roughly 1 hour, a donor bead solution was mixed with the reaction plates. This was followed by incubating reaction plates for roughly 1 hour at room temperature before being detected on the PHERAstar FSX, utilizing either an AlphaLISA SDE module (EmA: 615nm, EmB: 545nm) or a standard AlphaLISA module (Em: 615nm).
The PHERAstar FSX Instrument settings are listed in Table 1.
Table 1. The PHERAstar FSX Instrument settings
|
Focal height
|
8.0mm
|
|
Aperture spoon
|
384 well
|
|
Settling time
|
0.1s
|
|
Excitation time
|
0.26s
|
|
Integration start
|
0.28s
|
|
Integration time
|
0.12s
|
Results and Discussion
It was initially believed to be important to corroborate that the use of the AlphaLISA SDE module would not influence the well characterized AlphaLISA results. Figure 3 and Table 2 compare the performance of two modules used for detecting protein target 2 on separate plates. The results reveal that the performance of the SDE module is comparable to the conventional AlphaLISA® module.
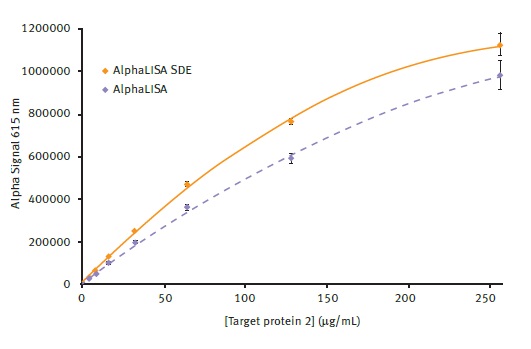
Figure 3. Comparison of AlphaLISA and AlphaLISA SDE module performance. Alpha signal is plotted vs. the different concentrations of target protein 2 present. Average signal from AlphaLISA and AlphaLISA SDE modules could be plotted using a 2nd order polynomial function with R2 values of 0.9986 and 0.9991 respectively. Error bars indicate standard deviation (n=8). Image credit: BMG Labtech.
Table 2. Assay parameters obtained either with the AlphaLISA standard optic module or with the AlphaLISA SDE optic module
|
|
AlphaLISA
|
AlphaLISA SDE
|
|
Signal/blank*
|
138.1
|
124.8
|
|
z-prime**
|
0.797
|
0.863
|
* Signal of protein at 256µg/ml divided by no protein control
** based on data for protein at 256µg/ml as positive control and no protein control as negative control
The next step was exploring the effect, if any, on detection due to the presence of a second signal in the well. For this purpose, target protein 1 dilutions were plated when target protein 2 was present, followed by reading the plates with the AlphaLISA SDE module. As can be seen in Figure 4, the detection of signal in the 545 channel is not affected in spite of the presence of a high level of signal in the 615 channel.
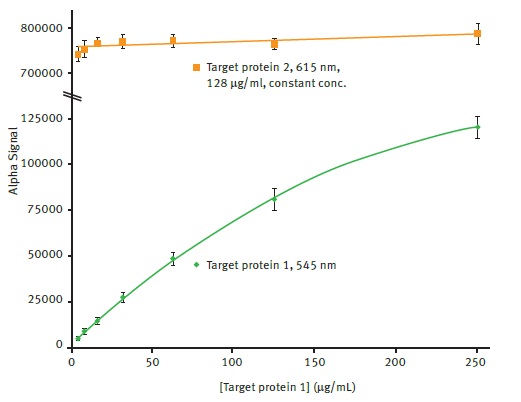
Figure 4. Comparison of AlphaLISA SDE module signal detection for 545nm and 615nm emission. Average 545nm Alpha signal is plotted vs. the different concentrations of target protein 1 present using a 2nd order polynomial function (R2 = 0.9997). Average 615nm Alpha signal is plotted for comparison. Error bars indicate standard deviation (n=8). Image credit: BMG Labtech.
Likewise, the any possible effect on the detection in the 615nm channel due to the presence of 545nm signal was explored. As can be observed in Figure 5, the detection of 615nm signal was not affected by the presence of 545nm signal.
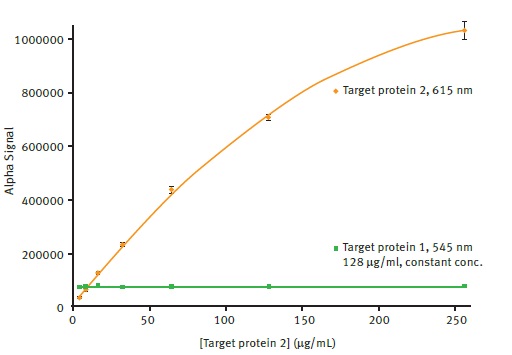
Figure 5. Comparison of AlphaLISA SDE module signal detection for 615nm and 545nm emission. Average 615nm Alpha signal is plotted vs. the different concentrations of target protein 2 present using a 2nd order polynomial function (R2 = 0.9997). Average 545nm Alpha signal is plotted for comparison. Error bars indicate standard deviation (n=8). Image credit: BMG Labtech.
Conclusion
The results have clearly demonstrated the ability of the PHERAstar FSX plate reader to perform simultaneous detection of both 615 and 545nm Alpha technology signals with no deterioration in data quality.
Acknowledgements
Produced from materials originally authored by Carl Peters, BMG Labtech, Cary, NC.
About BMG Labtech

BMG LABTECH has been committed to producing microplate readers for more than twenty years. By focusing on the needs of the scientific community, the company’s innovative microplate readers have earned the company the reputation of being a technology leader in the field.
BMG LABTECH has developed a wide range of dedicated and multi-mode microplate readers for life sciences applications and high-throughput screening.
All BMG LABTECH microplate readers are "Made in Germany" and are conceived, developed, assembled, and tested entirely at our headquarters in Germany.
Since our establishment in Offenburg, Germany in 1989, BMG LABTECH has expanded to offer a worldwide sales and support network with offices in the USA, UK, Australia, Japan and France. Our subsidiaries, regional offices and distributors are committed to bringing you innovative microplate reader technology with the quality and reliability you expect from a German company.
Our staff includes engineers and scientists from the fields of biology, biochemistry, analytical chemistry, and physics.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.