The HTRF technology invented by French company Cisbio has been developed further to provide kits, reagents and services for drug discovery research. In life science, the company’s homogeneous assays can be used to research kinases & signaling, biomarkers, epigenetics and G-protein coupled receptors (GPCRs).
The company offers different HTRF-certified, multidetection microplate readers such as the FLUOstar Omega, POLARstar Omega, CLARIOstar and PHERAstar. Microplate readers are only certified if they meet the Cisbio’s standards in terms of dynamic range, sensitivity, CV%, DF% and S/N.
This article describes the IP-One HTRF® assay from Cisbio carried out using two different HTS microplate readers with different detection technologies.
G-Protein Coupled Receptors
GPCRs are transmembrane proteins involved in the signal transduction of extracellular stimuli. They are associated with a complex arrangement of intracellular proteins controlling a wide range of downstream effectors. One such second messenger that is produced in response to the activation of Gq-coupled receptors, is inositol 1,4,5-triphosphate (IP3). However, owing to a very short half-life, IP3 is too difficult to assess in drug screening assays and instead, the release of calcium, triggered by IP3, has been widely used as a downstream readout of this signaling pathway. Another way to monitor IP3 is to measure the accumulation of a downstream metabolite of IP3 called inositol monophosphate (IP1).
Homogeneous time-resolved fluorescence assay (HTRF®) can be optimized by raising monoclonal antibodies against IP1, taking advantage of the stability of IP1 and its accumulation in cells. Comparison of HTRF® IP-One assay with existing methods has shown that it produces similar compound potency data. In addition, the end point accumulation of IP1 enables discrimination of slow acting compounds that cannot be observed by calcium sensing. Through the quantification of constitutively active GPCRs, the HTRF® IP-One assay also enables the characterization of inverse agonists which cannot be achieved by measuring calcium release. Furthermore, HTRF® IP-One detection confers superior assay robustness and significantly lower false positive rates when compared to calcium detection. The IP-One assay from Cisbio Bioassays is based on the proprietary HTRF® technology (Figure 1).
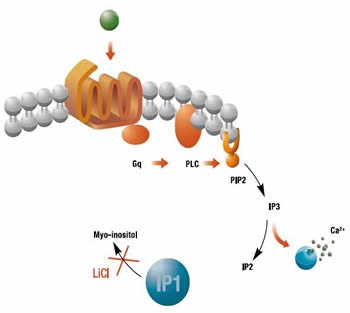
Figure 1. Biosynthesis of IP1. Image credits: BMG Labtech.
Principle of IP-One HTRF® Assay
The assay uses an IP1-specific monoclonal antibody and is based on a format where the fluorescence resonance energy transfer (FRET) signal between the HTRF® acceptor and donor is inhibited by the intracellular accumulation of IP1. An IP1 calibration curve can estimate the concentration of IP1 accumulated in cells as a function of the compound concentration.
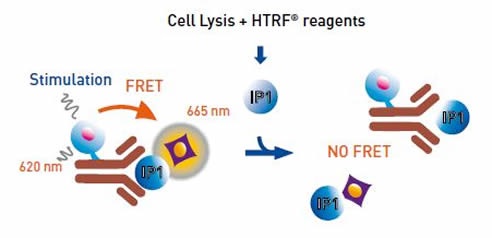
Figure 2. IP-One HTRF® Assay Principle. Image credits: BMG Labtech.
Materials and Methods
The following materials are used in the IP-One HTRF® assay experiment:
- HTS CCD-based microplate imager from a different vendor
- Next generation PMT based HTS microplate reader, PHERAstar FS
Black Greiner 1536-well microplates with an assay volume of 5μL were used. The last four columns of a microplate were filled with positive (POS, n=64) and negative (NEG, n=64) controls, and 1408 different compounds were pipetted into the first 44 columns, as shown in Figure 3.
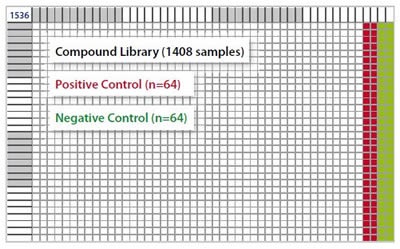
Figure 3. 1536-well microplate layout. Image credits: BMG Labtech.
The PHERAstar FS provides a dedicated simultaneous dual emission (SDE) direct photon counting time-resolved fluorescence mode and a high power pulsed nitrogen laser emitting at 337nm. The laser is superior to a broadband xenon flash lamp when exciting the terbium (Tb) donor molecule. The energy emission of the laser takes advantage of the higher molecular extinction coefficient of the terbium cryptate peaking at around 337nm, compared to europium (Eu).
Results and Discussion
Results were assessed using the HTRF® ratio of the two emission wavelengths (Em 665nm/ Em 620nm). A surface graph representing the entire 1536-well microplate shows the hits as peaks, as illustrated below in Figure 4 and Figure 5.
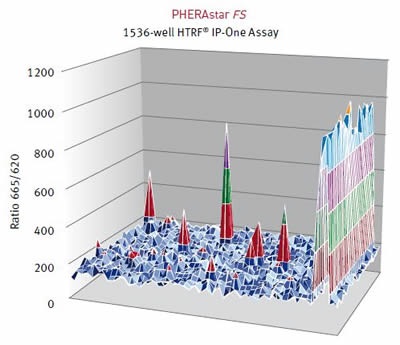
Figure 4. HTRF® ratios obtained for the IP-One assay with the PHERAstar FS. Image credits: BMG Labtech.
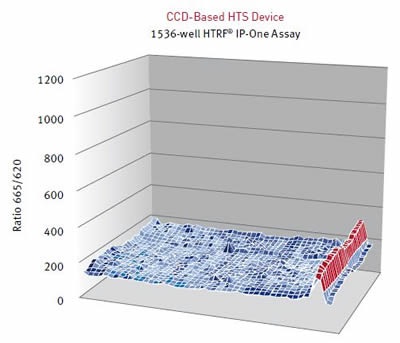
Figure 5. HTRF® ratios obtained for the IP-One assay with a CCD-based HTS Device. Image credits: BMG Labtech.
Table 1. Speed and assay quality are compared between the PHERAstar FS and the CCD camera based HTS reader.
|
|
PHERAstar FS
|
CCD based HTS reader
|
|
Read Times (1536)
|
53 sec
|
2:12 min
|
|
Assay Window
|
6:1
|
2:1
|
|
Delta F%
|
490
|
76
|
|
Z’ Value
|
0.70
|
0.24
|
Conclusion
Cisbio’s IP-One HTRF® assay was carried out on two different HTS microplate readers using different detection technologies. The PMT based PHERAstar FS can readily resolve low affinity compounds that cannot be detected using a leading HTS CCD camera-based imaging microplate reader.
The PHERAstar FS from BMG LABTECH is a next-generation HTS reader that serves as an ideal solution for HTS screening assays.

Figure 6. BMG LABTECH’s multidetection microplate reader PHERAstar FS. Image credits: BMG Labtech.
About BMG Labtech

BMG LABTECH has been committed to producing microplate readers for more than twenty years. By focusing on the needs of the scientific community, the company’s innovative microplate readers have earned the company the reputation of being a technology leader in the field.
BMG LABTECH has developed a wide range of dedicated and multi-mode microplate readers for life sciences applications and high-throughput screening.
All BMG LABTECH microplate readers are "Made in Germany" and are conceived, developed, assembled, and tested entirely at our headquarters in Germany.
Since our establishment in Offenburg, Germany in 1989, BMG LABTECH has expanded to offer a worldwide sales and support network with offices in the USA, UK, Australia, Japan and France. Our subsidiaries, regional offices and distributors are committed to bringing you innovative microplate reader technology with the quality and reliability you expect from a German company.
Our staff includes engineers and scientists from the fields of biology, biochemistry, analytical chemistry, and physics.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.