A universal regulatory mechanism behind protein activities is protein acetylation. Thanks to recent advances in epigenetic drug discovery, methods to investigate lysine deacetylation, such as in vitro assays of lysine deacetylases1, are particularly relevant.
In this article, Escherichia coli CobB’s enzymatic properties were established through the optimization of a commercial peptidase coupled lysine deacetylase assay. The sirtuin-type enzyme CobB plays a role in regulating bacterial chemotaxis, metabolism, translation, and transcription.2-4
Assay principle
The CycLex SIRT1 assay kit utilizes an acetylated peptide conjugated to a fluorophore and its quencher (panel 1). A functional peptidase cleavage site is generated when a lysine residue in the peptide (panel 2) is enzymatically deacetylated.
The fluorophore is separated from the quencher following the rapid cleavage of the deacetylated peptide by the peptidase, allowing fluorescent readout of the reaction (panel 3).
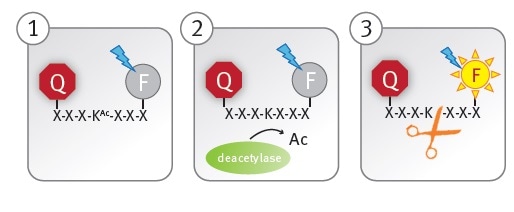
Figure 1. Schematic illustration of the lysine deacetylase assay principle. Image credits: BMG Labtech
Materials and methods
- 384-well black flat-bottom microplates (Greiner, #781900)
- CLARIOstar microplate reader from BMG LABTECH
- Recombinant Escherichia coli CobB
- SIRT1 fluorimetric assay kit (CycLex, CY-1151V2)
All standard disposables and chemicals were received via normal distribution channels. A pET28 expression vector-transfected E.coli BL21(DE3) strain was used to express His6-tagged E.coli CobB.
The enzyme was purified by standard methods involving size exclusion chromatography (Superdex-75, GE Healthcare) and immobilized metal ion chromatography (HisTrap, GE Healthcare).
CobB lysine deacetylase activity measurements were performed at an ambient temperature of around 21°C under the conditions outlined in the manufacturer’s protocol, with modifications as explained below. 50 nM CobB, NAD+ and inhibitor were used as specified to perform the enzymatic assays.
Instrument settings
|
Detection Mode
|
Fluorescence Intensity
|
|
Detection Method
|
Plate Mode Kinetic
|
|
No. of cycles
|
120
|
|
Cycle time (sec)
|
60
|
|
No. of flashes
|
5
|
|
Scan mode
|
orbital averaging
|
|
Scan diameter (mm)
|
1
|
|
Excitation
|
355-10
|
|
Dichroic
|
400 (autoset)
|
|
Emission
|
445-10
|
|
Shaking
|
8 seconds before each cycle
|
|
Shaking mode
|
orbital, 400 rpm
|
Results and discussion
Optimization of excitation and emission parameters
It is possible to improve the emission and excitation settings as indicated by spectral scans (Figure 2) of the fluorogenic peptide provided with the assay kit.
The CLARIOstar monochromators with emission at 445/10 nm, excitation at 355/10 nm, and a dichroic set at 400 nm were used in this study. The settings provided the highest signal without changing the Z’ factor5, which was found to be 0.78 using the above conditions or the conditions recommended by the provider.
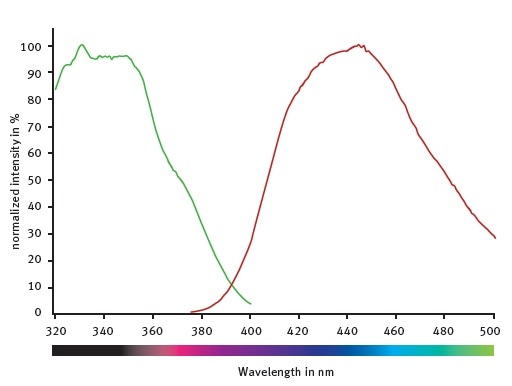
Figure 2. Excitation and emission spectra of the fluorogenic peptide provided with the SIRT1 assay kit. Image credits: BMG Labtech
Reference curve for kinetic measurements
The measurements were calibrated using a dilution series of the deacetylated reference peptide featured in the assay kit (not shown).
Basic kinetic parameters of CobB lysine deacetylase activity
CobB enzymatic assays were performed in the presence of different NAD+ concentrations and fluorogenic substrate peptide at the concentration recommended by the SIRT1 assay kit manual. A KMNAD+ of 71 μM was determined under the experimental conditions (Figure 3). The researchers are not aware of the reported kinetic parameters for NAD+ cleavage by CobB.
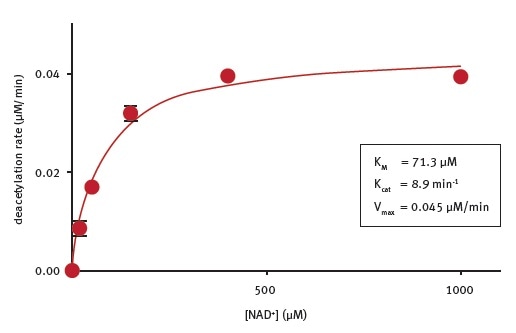
Figure 3. Determination of the enzymatic properties of E.coli CobB. Image credits: BMG Labtech
Inhibition of CobB by a general sirtuin inhibitor
The potency of Ex527, a general sirtuin inhibitor6, for inhibiting CobB activity was determined in this study. To perform the assays, the researchers used 70 μM NAD+ as co-substrate and Ex527 at varying concentrations, with a final concentration of DMSO of 1%. Figure 4 shows the results of this analysis.
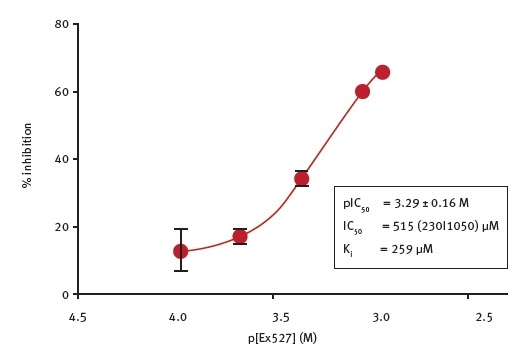
Figure 4. Determination of the potency of Ex527 inhibition of E.coli CobB. Image credits: BMG Labtech
Ex527 is a weak inhibitor of CobB with an IC50 value of 515 μM and Ki value of 259 μM (calculated using the Cheng-Prusoff equation). The quality of the determination was compromised due to the low potency, limited solubility of the inhibitor and the DMSO sensitivity of the enzyme. At 1% DMSO, the effect of Ex527 at concentrations more than 1 mM could not be determined.
Conclusion
In this study, E.coli lysine deacetylase CobB’s kinetic properties as well as its inhibition by a common inhibitor of sirtuins were determined using a commercially available assay kit with the help of the CLARIOstar multimode microplate reader.
The study results demonstrate a general method that can be used for similar kind of analyses into other lysine deacetylases and, in particular, lysine directed acetyltransferases.
Acknowledgments
The CobB expression vector was kindly provided by Dmitry Ivanov (A-Star Institute of Molecular and Cell Biology, Singapore). This work was supported by the Swedish Foundation for Strategic Research, the IngaBritt and Arne Lundbergs Research Foundation, and Karolinska Institutet.
Produced from materials originally authored by M. Rajaee, T. Ekblad and H. Schüler Karolinska Institutet from the Department of Medical Biochemistry and Biophysics, Stockholm, Sweden
References
- Huston et al. (2015) Nat. Chem. Biol. 11, 542-545.
- Baeza et al. (2014) J. Biol. Chem. 289, 21326-21338.
- Thao et al. (2010) PLoS One.5(12), e15123.
- Li et al. (2010) Mol. Microbiol. (76), 1162-1174.
- Zhang et al. (1999) J. Biomol. Screen. (4), 67-73.
- Ekblad & Schüler (2016) Chem. Biol. Drug Des. 87, 478- 482.
About BMG Labtech

BMG LABTECH has been committed to producing microplate readers for more than twenty years. By focusing on the needs of the scientific community, the company’s innovative microplate readers have earned the company the reputation of being a technology leader in the field.
BMG LABTECH has developed a wide range of dedicated and multi-mode microplate readers for life sciences applications and high-throughput screening.
All BMG LABTECH microplate readers are "Made in Germany" and are conceived, developed, assembled, and tested entirely at our headquarters in Germany.
Since our establishment in Offenburg, Germany in 1989, BMG LABTECH has expanded to offer a worldwide sales and support network with offices in the USA, UK, Australia, Japan and France. Our subsidiaries, regional offices and distributors are committed to bringing you innovative microplate reader technology with the quality and reliability you expect from a German company.
Our staff includes engineers and scientists from the fields of biology, biochemistry, analytical chemistry, and physics.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.