Inappropriate regulation of insulin secretion can result in extensive metabolic problems, eventually causing diabetes mellitus1. It is extremely important that insulin is directly measured for studies elucidating the mechanism of glucose stimulated release as well as the impact of dopaminergic modulators of insulin release2.
In this article, a rapid insulin detection assay is developed based on the homogeneous time resolved fluorescence (HTRF) detection technique (Figure 1), using the PHERAstar FS microplate reader. The technology featured in the PHERAstar FS is designed for optimization of HTRF detection, and assisting the detection of HTRF assay.
Rapid and cost-effective results can be achieved using this HTRF insulin assay. The assay can also detect insulin from various species. Tests were carried out using insulin secreting tissues and cell lines in order to confirm the performance of this assay. The test results confirm the suitability of this system to detect the impact of modulators on the secretion of insulin.
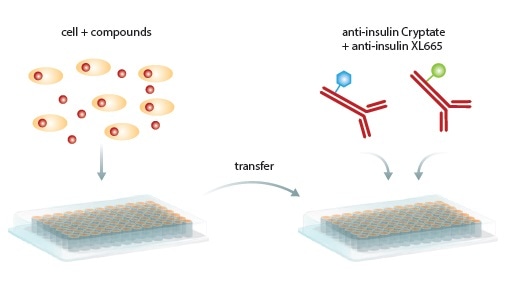
Figure 1. Homogeneous HTRF Assay procedure. Secreted insulin in cells or supernatant is transferred to a new microplate, antibodies are added, incubated and read by the PHERAstar FS microplate reader. Image credits: BMG Labtech.
Assay principle
The HTRF insulin assay is based on two insulin binding antibodies (Figure 2). A Europium cryptate donor is used to label one of the antibodies and a near-infrared acceptor for the second antibody.
Once these antibodies are attached to insulin, they are within the proximity for fluorescence resonance energy transfer (FRET) to occur. Owing to the nature of the donor, it is possible to set a time delay before initiating measurements, reducing the transient interference caused by other assay components.
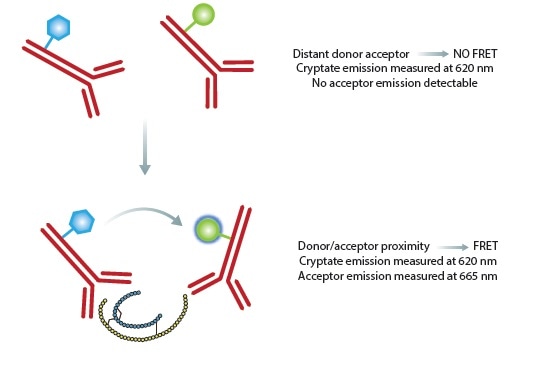
Figure 2. Principle of the HTRF insulin assay. In the absence of insulin, no FRET is occurring and only the donor will give a signal. The binding of both antibodies to insulin results in physical proximity, FRET occurs and acceptor emission can be detected. Ratiometric measurements reduce well to well variation. Image credits: BMG Labtech.
Materials and methods
- 96-well (half area) or 384-well, white plates (Greiner)
- PHERAstar FS microplate reader
- Rodent insulin (ALPCO)
- Bromocriptine mesylate (Tocris)
- Anti-insulin: Eu and anti-insulin: XL665 (Cisbio)
All other compounds were procured from SigmaAldrich. Information about cell culture, mouse pancreatic islet isolation, and treatment of cells and tissues with insulin secretion modifying compounds can be found in Farino and Morgenstern et al.2
Both anti-insulin antibodies were added to samples at a ratio of 1:1 (total antibody volume: sample volume) to perform the HTRF insulin detection assay. The microplate was incubated for 2 hours in pH 7 buffer at 25 °C and subsequently read on the PHERAstar FS.
PHERAstar FS instrument settings
|
Measurement type
|
Time Resolved Fluorescence
|
|
Measurement mode
|
endpoint
|
|
Optic module
|
HTRF (337/665/620)
|
|
Focal height
|
adjust prior to test run
|
|
Integration start
|
40 μs
|
|
Integration time
|
100 μs
|
|
Excitation source
|
flash lamp
|
|
Flashes/well
|
100
|
Results and discussion
The ability to detect insulin using the HTRF insulin assay from a range of species was tested (Figure 3). The test results reveal the suitability of the assay for use with a variety of animal models and human cells.
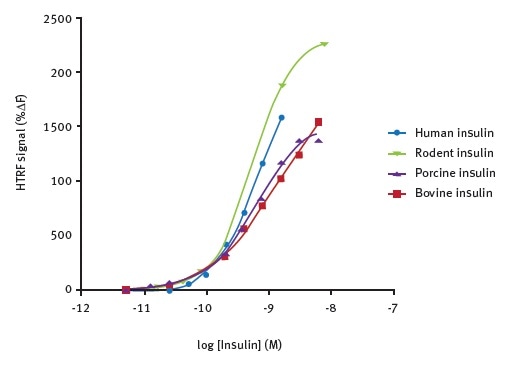
Figure 3. Species cross-reactivity of antibodies. Data taken from Farino et al.2
The HTRF signal did not vary significantly during the testing of insulin from cows, pigs, rodents, or humans across a wide concentration rage of 0.01 – 10 nM (n = 3).
The Z’ – factor was then determined to assess the suitably of this HTRF insulin assay for high throughput screening. The well-to-well variation of standards at various insulin concentrations is shown in Figure 4.
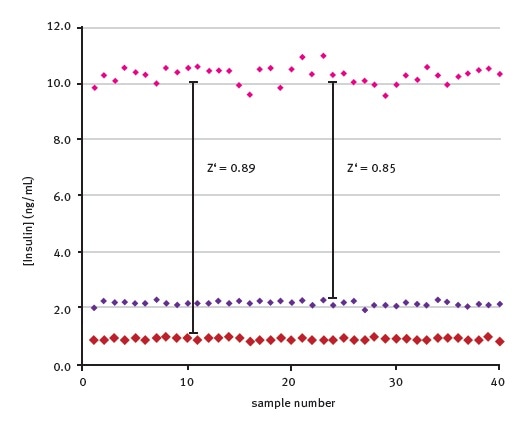
Figure 4. Well-to-well variation of back calculated insulin concentrations. Data was first published in reference 3.
Results obtained are based on 40 replicates, confirming the suitability of this assay for high throughput screening applications (the Z´ value, calculated between a concentration range of 2.5-10 ng/ml insulin samples, is 0.85).
The potential of this insulin assay was further assessed by testing the hypothesis that dopamine D2/D3 receptor agonist called bromocriptine might directly act on pancreatic islets. The test results (Figure 5) confirm the ability of bromocriptine to inhibit glucose-stimulated secretion of insulin in mouse pancreatic islets.
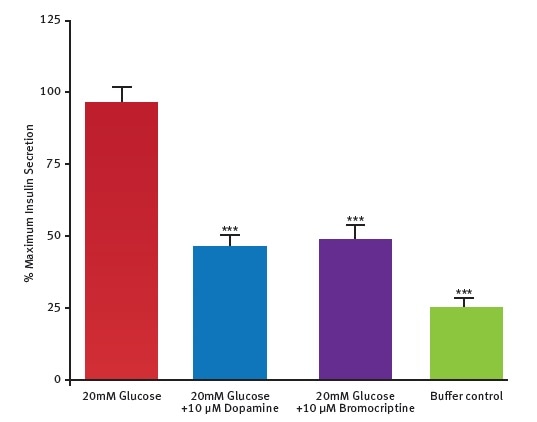
Figure 5. Measurement of bromocriptine effect on insulin production in islets. Data are presented as % maximal insulin secretion in response to glucose. Bromocriptine inhibited insulin secretion by 67.4% ± 8.1% (n = 6). From Farino et al.2
Conclusion
The HTRF insulin assay is rapid and simple to perform. The study results discussed in this article reveal that this homogenous assay is ideal for medium and high throughput. In addition, the cost involved in performing this assay is very low ($ 0.02 / sample)2.
Acknowledgments
Produced from materials originally authored by Z.J. Farino1 , T.J. Morgenstern1 , J. Vallaghe2 , N. Gregor2 , P. Donthamsetti1 , P.E. Harris1 , N. Pierre2 , R. Freyberg3 , F. Charrier-Savournin2 , J.A. Javitch1 and Zachary Freyberg1 from:
1 Columbia University, NY
2 Research Dept., Cisbio Assays, France
3 Yeshiva University, NY
References
- Kahn SE et al. (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes Nature 444:840- 846.
- Farino ZJ and Morgenstern TJ et al. (2016) Development of a Rapid Insulin Assay by Homogenous Time-Resolved Fluorescence PLoS ONE 11:e0148684.
- Claret EJ et al. (2004) A high-throughput HTRF assay for human/rat insulin. Cisbio International. SBS Poster.
About BMG Labtech

BMG LABTECH has been committed to producing microplate readers for more than twenty years. By focusing on the needs of the scientific community, the company’s innovative microplate readers have earned the company the reputation of being a technology leader in the field.
BMG LABTECH has developed a wide range of dedicated and multi-mode microplate readers for life sciences applications and high-throughput screening.
All BMG LABTECH microplate readers are "Made in Germany" and are conceived, developed, assembled, and tested entirely at our headquarters in Germany.
Since our establishment in Offenburg, Germany in 1989, BMG LABTECH has expanded to offer a worldwide sales and support network with offices in the USA, UK, Australia, Japan and France. Our subsidiaries, regional offices and distributors are committed to bringing you innovative microplate reader technology with the quality and reliability you expect from a German company.
Our staff includes engineers and scientists from the fields of biology, biochemistry, analytical chemistry, and physics.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.