Reactive oxygen species are unstable molecules containing oxygen as a by-product of the natural metabolism of oxygen. They are very reactive and can take many different forms including NO (nitric oxide), peroxynitrite (ONOO-), H2O2 (hydrogen peroxide), hydroxyl radical (OH-) and hydrochlorous acid (HOCl)1.
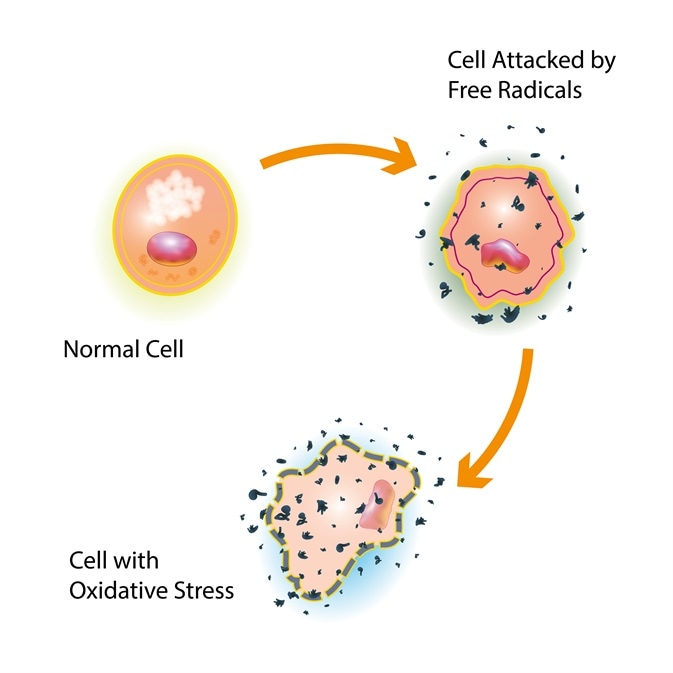 Image credits: Shutterstock | CLUSTERX
Image credits: Shutterstock | CLUSTERX
The build-up of reactive oxygen species to a high level inside cells is known as oxidative stress. This causes damage to proteins, DNA and RNA and can even result in cell death. ROS biomolecules have been implicated in a variety of pathologies (disease). These include neurodegenerative diseases, cancer, the ageing process and atherosclerosis2. In response to oxidative stress, the body increases production of antioxidants, such as glutathione and catalase, and these convert the dangerous free radicals into harmless molecules like water3.
However, reactive oxygen species in low levels have been identified as having useful and beneficial effects. For example, they have an important role in gene expression and the regulation of cellular signaling. There is also evidence to suggest that ROS may have a role in the modulation of cell differentiation, including the hemopoietic differentiation involving stem cell production, in both pathological and physiological conditions4.
Reactive oxygen species are produced from many intracellular processes including from cell mitochondria and NADPH oxidases (NOX enzymes) linked to neutrophils2. Neutrophils are white blood cells which produce high levels of ROS as part of their defense role. ROS is also produced by a broad variety of enzymes, including nitric oxide synthase, xanthine oxidase, lipoxygenases, cyclooxygenases and cytochrome P450 enzymes.
Exogenous stimuli further induce oxidative stress, for example alcohol leads to formation of reactive oxygen species during its degradation and activates cytochrome P450 enzymes which induce ROS production5. Tobacco smoke also induces ROS because it contains free radicals that react with oxygen to form ROS6. Ultraviolet light is also an exogenous inducer but is less avoidable than alcohol or tobacco smoke. Inducing reactive oxygen species increases the oxidative damage to cellular components and therefore contributes to the development of skin cancer7.
Assays Detecting Reactive Oxygen Species
Reactive oxygen species are studied intensely because they impact on many biological events. Assays that measure ROS are used to monitor the balance of ROS and antioxidants, to study their role in cancer, or to report on immune responses. However, it can be difficult to detect ROS levels because they have very short half-lives and are often in the nanomolar range.
Most commonly, the reagent used for measuring ROS is 2',7'-dichlorodihydrofluorescein diacetate (H2DCFDA). It is trapped intracellularly by esterase activity that removes lipophilic blocking groups. The resulting dichlorofluorescein (DCF) exhibits high fluorescence when the probe is oxidized by ROS. Different derivatives of H2DCFDA have specific characteristics so they are used for ROS measurements. The fluorinated molecule (H2DFFDA) proves more stable when exposed to light whereas carboxy-H2DCFDA displays an improved cellular retention.
The MitoSOXTM Red (ThermoFisher) is a probe that selectively detects superoxide generated in mitochondria during oxidative phosphorylation. The live cell reagent is targeted to mitochondria and its oxidation by superoxide increases its red fluorescence.
Changes in reactive oxygen species directly in the cell at the location of interest are reported by genetically encoded probes. Depending on the redox state, the fluorescent probe roGFP (redox sensitive green fluorescent protein) has different excitation wavelengths. Oxidized roGFP is excited at 405 nm whereas reduced roGFP is better excited at 488 nm. The oxidation of roGFP is facilitated by a peroxidase that selectively scavenges H2O2 and the presence of H2O2 is reported by the ratio of measurements performed with both excitation wavelengths8. The genetically encoded H2O2 sensor HyPer employs a similar approach.
Luminescent methods are available as well as fluorescent methods in order to detect reactive oxygen species. These methods can be based on chemiluminescence. They use a molecule such as lucigenin or luminol that emits light when transitioning to the oxidized state. This process is induced by ROS and so light emission can be directly translated to the presence of ROS9.
Enzyme-dependent luminescence (bioluminescence) is another method used to detect ROS. The enzyme used is a luciferase, which converts a substrate when under light emission. A substrate precursor is added to the sample and is ROS-dependently converted into the luciferase substrate. Therefore, the luminescent signal correlates directly with ROS levels10.
Instrumentation Required for Detecting Reactive Oxygen Species
These common methods to study and measure levels of ROS are based on either luminescent or fluorescent read-outs. Therefore, it is possible to use a microplate format which allows 96 to 1563 samples to be run simultaneously and for the detection to be performed in the microplate readers. The devices record light signals in the tiny reactions that take place in the microplate wells.
Detection modes: depending on the assay chosen to detects ROS, the ideal microplate reader needs either to cover fluorescence, luminescence or both if the flexibility running different assays should be given. However, further detection modes may be need to be covered if it is important to combine the ROS-measurement with other assays. For example, combining ROS-measurements with absorbance-based viability assays, e.g. WST. BMG LABTECH provides a variety of multi-mode plates readers that are capable of measuring luminescence, fluorescence, absorbance and advanced detection modes.
Sensitivity: The plate format used and the ROS-assay dictate the sensitivity required by the ideal microplate reader. For example, high density plate formats (1536 well) have lower signal intensities and a lower reaction volume. This means that the microplate reader must be highly sensitive and read the plate quickly. The PHERAstar FSX microplate reader is designed to meet these specific requirements.
When used in the 96-well or 384-well format with sufficient material, the H2DCFDA assay, the most commonly used ROS-assay, gives high signal intensities. An example of this application is when using immortal cell lines. Signals can be recorded easily by all BMG LABTECH multi-mode readers. Since expression levels are limited, genetically encoded probes, such as roGFP, require increased sensitivity. The challenge of reading the fluorescent probe and how the CLARIOstar® assisted the measurements are explained in detail by researcher Prince Saforo Amponsah from the Technical University Kaiserslautern (https://www.bmglabtech.com/de/clariostar-review2018-1/).
Accessories: Detection of reactive oxygen species based on bioluminescence, roGFP or H2DCFDA is suitable to be kinetically measured in live cells. Live cell measurements require gas atmosphere to allow long-term cell incubation, depending on the time frame of interest. This is a CO2 atmosphere of 5-10% which ensures a stable pH in carbonate buffered media.
O2 concentration plays a critical role in the measurement of ROS. An atmospheric level of 21% is capable of inducing oxidative stress without any further stimulation. Physiologic O2 concentrations, which cells are exposed to in the human body, are much lower and range from 4% in the brain to 10% in the kidney11. Both the CO2 and O2 concentrations in the microplate reader are regulated by the atmospheric control unit (ACU) so that ROS experiments can be run at physiological O2 levels. This tool is available for Omega and CLARIOstar microplate readers.
References
- https://www.cancer.gov/publications/dictionaries/cancer-terms/def/reactive-oxygen-species
- Holmström & Finkel. (2014) Cellular mechanisms and physiological consequences of redox-dependent signaling. Nature Reviews, Molecular Cell Biology (15) 411.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3308605/
- https://www.sciencedirect.com/science/article/pii/S1040842811000722
- http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.535.2817&rep=rep1&type=pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2672368/
- https://www.ncbi.nlm.nih.gov/pubmed/9426184
- http://www.jbc.org/content/284/46/31532.long
- https://www.bmglabtech.com/fileadmin/06_Support/Download_Documents/Application_Notes/AN262.pdf
- https://www.promega.de/products/cell-health-assays/oxidative-stress-assays/ros_glo-h2o2-assay/?catNum=G8820
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4373326/
About BMG Labtech

BMG LABTECH has been committed to producing microplate readers for more than twenty years. By focusing on the needs of the scientific community, the company’s innovative microplate readers have earned the company the reputation of being a technology leader in the field.
BMG LABTECH has developed a wide range of dedicated and multi-mode microplate readers for life sciences applications and high-throughput screening.
All BMG LABTECH microplate readers are "Made in Germany" and are conceived, developed, assembled, and tested entirely at our headquarters in Germany.
Since our establishment in Offenburg, Germany in 1989, BMG LABTECH has expanded to offer a worldwide sales and support network with offices in the USA, UK, Australia, Japan and France. Our subsidiaries, regional offices and distributors are committed to bringing you innovative microplate reader technology with the quality and reliability you expect from a German company.
Our staff includes engineers and scientists from the fields of biology, biochemistry, analytical chemistry, and physics.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.