Currently in developed countries, life expectancy is far higher than 80 years of age. However, with a longer life expectancy comes increased chances of developing a neurodegenerative disease, like Alzheimer’s disease, Parkinson’s disease and dementia. The progression of these disorders result in an increasingly debilitating situation until full-time care is needed.
Unfortunately, the etiology of these diseases is still not completely understood, and the majority of medication only treats symptoms. With life expectancy increasing, it is essential to enhance our understanding of these diseases and develop novel therapies.
Biological assays, including aggregation, cytotoxicity and signaling assays, are vital in aiding researchers with their understanding of the underlying mechanisms of these conditions, in addition to discovering promising treatments in the primary stages of drug development.
This article explains the importance of these assays and the requirement for sensitive and rapid instruments that are able to analyze assays with a variety of detection techniques.
What are neurodegenerative diseases?
Neurodegenerative is a term that refers to diseases that are usually recognized by symptoms like decreased motor control, mood disorders and cognitive deficits. As indicated by the name, the conditions are characterized by degeneration of the neurons. Neurons are nerve cells in the brain and spinal cord that do not replicate.
If neurons become damaged they are unable to recover, resulting in brain dysfunction and incurable diseases. Despite there being similarities between neurodegeneration in the related conditions, the origin of the causes may differ.
For example, Huntington’s disease is triggered by a gene mutation, Creutzfeldt Jakob prion disease is caused by inaccurately folded protein (prion), and Parkinson's and Alzheimer's are suggested to be caused by a genetic and environmental factor combination and usually occur in individuals over the age of 60.
A similar feature among these neurodegenerative conditions is how damage is induced in the neurons and thus, the brain. Incorrectly folded proteins occur in the progression of all the mentioned conditions.
Amyloid fibrils are formed and protein aggregates resulting in a build-up of plaques, potentially harming neuronal cells and brain tissue. Understanding and disturbing this alteration is an essential approach for treating neurodegeneration as it may be an advantage to patients with any of the conditions.
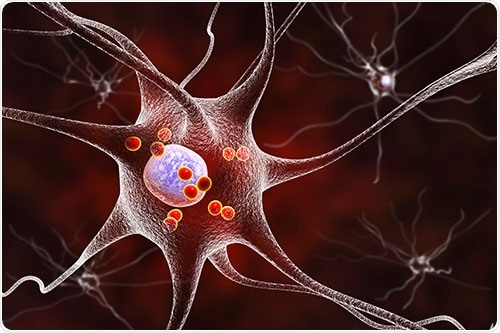
Figure 1: Nerve cells (neurons) are the target of neurodegeneration. Loss of neurons and neuronal function is due to abnormal protein aggregation or reduced signaling. In Parkinson's Disease, protein accumulations of alpha-synuclein occur inside the cell (Lewy Bodies, red in the picture) and disrupt neuron function. Image Credit: BMG Labtech
Why is neurodegenerative disease research so important?
Neurodegenerative diseases mimic cardiovascular diseases and cancer as main causes of death. Unlike cardiovascular diseases and cancer, neurodegenerative diseases are unable to be cured or their progression slowed significantly.
Nerve disorders symptoms are impaired movement, memory loss, mood variations, impaired speaking and several more.
To begin with, these dysfunctions do not impair patients to a significant level and they can continue to live an independent life. However, as the progression of the diseases continue, the quality of life of the patients is reduced dramatically until they are in need of full-time care. As a consequence, there is a social and financial burden during disease progression too.
Currently in Europe, roughly €130 billion is spent per year to care for individuals with dementia, a consequence of neurodegenerative conditions. With increases seen in the aging population, the amount of affected people also increases.
In 2015, Alzheimer’s Disease impacted 40 million people globally, and this is estimated to rise to 130 million by 2030, with one-third to one-half of people over the age of 85 years developing this disease. The average duration of Alzheimer’s Disease is 2 to 10 years, which undoubtedly has an effect on the predicted economic toll in the USA, of $1 trillion per annum, of this disease by 2050.
To date, there are a limited amount of treatments that are used for neurodegenerative diseases, and the majority of these just treat symptoms. Actually, there has been no approval of novel drugs for Alzheimer’s Disease in Europe in the last five years.
With this in mind, successful therapies to delay or lessen the symptoms of these debilitating conditions is vital in order to limit the detrimental influence it has on individuals, families, societies and economies.
A delay in the onset of Alzheimer’s Disease by only five years would lessen the financial burden in the USA by 50% and shows that even a small delay would be an advantage, allowing improved independence of the patient and relief to the family and their commitments and the public health bill.
Drug target identification for neurodegenerative diseases
For the identification of possible new drugs for the treatment of neurodegenerative disorders, it is practical to gain an understanding of the disease itself. Broad and differing symptoms and characteristic slow onset, means that diagnosis is frequently only made when the disease is already progressing.
With this in mind, it is still evident that there remain gaps in the understanding of these disorders, specifically the triggers and initial stages of the conditions. Consequently, fundamental research is ongoing to give a more detailed understanding of these diseases and aid in the identification of novel drugs.
In the last 10 years, the development of new assays has given potential to the study of neurodegeneration processes in vitro and in high throughput. This provides the potential to test numerous experimental conditions or numerous potential drugs in extremely short times.
Many key assays are valuable tools for the analysis of the disease pathway and assessing the effects of possibly drugs. Following this, assays testing aggregation, cytotoxicity, signaling and protein quantity is explained and demonstrates how they aid in the understanding of neurodegenerative diseases.
Neurodegenerative disease studies: Aggregation assays
A main feature of neurodegenerative diseases is the creation of soluble, functional proteins into insoluble, highly ordered protein aggregates called amyloid fibrils or plaques. This transition starts with prefibrillar species (dimers, tetramers, hexamers etc.) forming before progressing into large protein aggregate complexes.
With regards to prions, traditionally, whole animal models have been utilized to monitor these protein aggregates via long bioassays that may take up to 6 months. In 2012, at Rocky Mountain Laboratories in Montana, a quicker, higher throughput aggregation assay for prion seeding monitoring was developed by investigators. It was called real-time quaking induced conversion assay (RT-QuIC).
RT-QuIC uses fluorescence intensity for the measurement of the aggregation of prion proteins. The fluorescent dye, thioflavin T (ThT), is added to recombinant proteins and the molecule binds to beta-sheets created during fibril formation, which induces a fluorescence increase. The technique is completed in microplates and uses recombinant prion protein, tau protein or alpha-synuclein. The plate is sporadically shaken to allow the breaking up of fibrils during shaking and new fibril formation during quiescence.
The time it takes to complete the method is 168 hours, but fibrils formation is increased at higher temperatures. Due to this, the protein aggregation assay is often completed at 37 °C or higher. Combining high temperature, intermittent shaking and extended run times puts high demands on the measuring equipment.
The Omega microplate reader series has shown evidence to be robust and reliable to complete the tedious work as summarized in the application note; “Real-time quaking induced conversion assay for prion seeding”.
The Omegas have been selected as the readers of choice for aggregation assays due to their high temperature incubation (up to 65 °C), enhanced plate carrier and an increasingly robust transport system. The webinar demonstrates tips and tricks for full optimization of the RT-QuiC measurements.
Optimizing sensitivity and specifity in the RT- QUIC assay
Video Credit: BMG Labtech
Research published in 2016 found the RT-QuiC performed with cerebrospinal fluid to be a reliable test for sporadic Creutzfeldt Jakob disease. Eleven centers based in Europe, Asia and Australia analyzed the human CSF samples with RT-QuiC and BMG LABTECH plate readers (with one exception). A diagnosis of Creutzfeldt Jakob disease from the centers occurred with 100 % accordance (McGuire et al. 2016).
A technique appropriate for monitoring the onset of aggregation uses a novel fluorophore: bis(triphenyl phosphonium) tetraphenylethene (TPE-TPP). The dye has superior properties to the ThT dye as it is more efficient at binding. Additionally, the monitoring of its fluorescence polarization (FP) shows on early stages (dimers, tetramers) of aggregation.
FP measures the rotation of molecules in solution. Small molecules move rapidly and depolarize emission whereas large molecules move slower and keep emission polarization. The binding of TPE-TPP to (pre-)amyloid structures reduces the speed of its rotation resulting in measurable changes in FP.
The assay was developed by researchers located in Australia which utilized a CLARIOstar microplate reader to complete the FP measurement. An in depth assay explanation can be found in the Application Note: “Novel aggregation-specific fluorogen monitors prefibrillar protein aggregation by fluorescence polarisation (FP)”.
Viability and toxicity assays: Better understanding and the modification of neuronal death
Apart from protein aggregation, neuronal cell death is a hallmark of neurodegeneration. While in healthy adults neurons are restricted in cell death, during neurodegenerative diseases they die resulting in loss of brain function. The neurons die through mechanisms of programmed cell death which can be caused by oxidative damage of mitochondria or DNA, damage to the membrane by protein aggregates or others.
Programmed cell death mechanisms seen in neuronal cells are apoptosis, necrosis and autophagy. Enhancing understanding of the causes of neuronal cell death, its mechanisms and how to inhibit it is a method for discovering medication for neurodegenerative diseases. Several assays study cell death and aid in deciphering and modulating neuronal cell death. An overview of basic cytotoxicity assays is demonstrated in the blog post: “Cytotoxicity – These assays tell you what your cells don’t like”.
The most frequently utilized cytotoxicity assays are formed on the basis of tetrazolium salts. Their decrease in viable cells results in an absorbance shift that is recorded by microplate readers and indicates metabolic activity. Investigators at the National Medical Research Center, Moscow, Russia used the assay to give evidence that insulin limits excitotoxicity in cortical neurons, an effect causing cell death in neurons that is associated with neurodegenerative diseases (Krasil’nikova et al. 2019).
Due to their sensitivity and simplicity, ATP-dependent luciferase viability assays are popular. They measure the ATP content of lysed cells by an ATP-dependent luciferase. The light output measured by a microplate reader is directly linked to cell number. The assay aided researchers in Singapore to uncover a cytotoxic effect of mononamine oxidase (MAO) activity in a huntingtin cell model (Ooi et al. 2015).
Newly developed cell death assays report on toxicity at one timepoint and also monitor cytotoxic effects over time. The RealTime-Glo™ viability assay generates light when viable cells are present. If cells are maintained in a culturing environment (37 °C, 5 % CO2) by an atmospheric control unit regulating gases within the reader, it is possible to use it for real-time viability monitoring over days.
The novel Atmospheric Control Unit (ACU) for the CLARIOstar offers adaptability in long-term cell-based assays. Utilizing the assay on a CLARIOstar microplate reader, the study of a protective effect of a specific fusion protein against neurotoxins was completed (Paliga et al. 2019).
Signaling assays and neuron function
An early feature of neurodegeneration is weakened neuron signaling. Altered signaling processes include Ca2+ signaling regulating synaptic transmission, energy metabolism and cell survival or signal transmission via receptors.
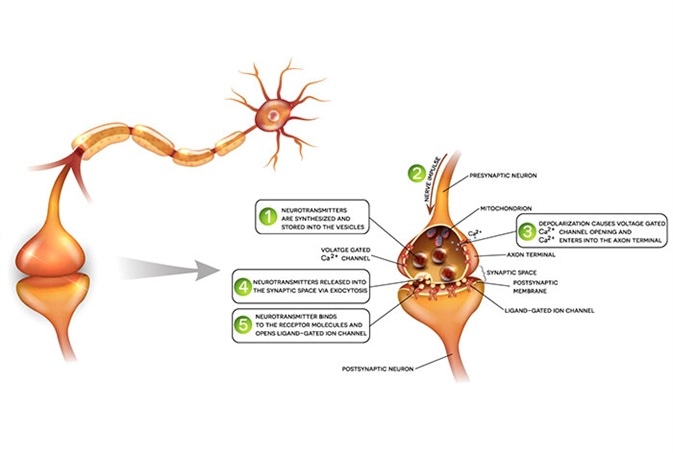
Figure 2: Signal transmission between neurons includes signaling via neurotransmitters and receptors, ions and ion channels and induces intracellular calcium responses. Impaired signaling occurs in neurodegenerative diseases and reversing functional signaling is a therapeutic approach. Image Credit: BMG Labtech
Ca2+ signaling in neurons is essential for their cellular function as transmitters of information. Intracellular Ca2+ variations can be monitored using fluorescent dyes like Fura-2, Fluo-8 or Cal- 520. The application note: “Monitoring intracellular calcium using fluorescent dyes in a mid-throughput assay” is a comparison of the different assays monitoring Ca2+.
One instance of Ca2+ assays aiding in neurodegenerative diseases studies is the use of Fura-2 to develop a model of differentiated neurons that are superior in reflecting neurons compared to the human neuroblastoma cells they are derived from (Ferguson et al. 2016). Fura-2 intracellular Ca2+ measurements demonstrated the stimulation of differentiated cells with KCl, while non-differentiated cells did not increase intracellular Ca2+ levels upon KCl.
Neurodegeneration biomarker quantification
A number of proteins have been discovered to demonstrate changed expression during the different neurodegenerative diseases. In Alzheimer’s disease, Tau protein is implicated, the TREM2 receptor levels correlate with the risk to develop Alzheimer’s, and the BDNF growth factor is reduced in Alzheimer’s. For Parkinson’s disease, higher levels of neuroinflammation like IL6, IL β1 and TNF α are observed.
A popular technique for the quantification of certain proteins in solution is ELISA assays. The application note: “Fast and accurate detection of Alzheimer’s Disease targets with SimpleStep ELISA® kits and SPECTROstar® Nano” describes the ELISA principle and demonstrates how it was utilized to quantify proteins linked to neurodegeneration.
Researchers based in Sydney, Australia utilized a TNF-alpha ELISA to examine the effect of chronic microglial activation on the inflammatory marker. They discovered pro-inflammatory TNF-alpha to be raised at all ages of a mouse model with chronic microglial activation, a characteristic of neurodegenerative diseases (Gyengesi et al. 2019).
Neurodegeneration research instrumentation
This research shows high throughput techniques utilized in studies with neurodegenerative diseases as a key focus. As numerous detection modes are required, the use of multimode plate readers is suggested for neurodegenerative disease research.
For the performance of aggregation assays, it is vital that the analysis instrumentation can shake and incubate microplates over extended periods of time, up to 7 days. BMG Labtech are technology leaders in the microplate field. The multimodal plate readers include the CLARIOstar® Plus and Omega series, which include a dedicated plate carrier and offer strength in long-term shaking aggregation measurements.
Acknowledgments
Produced from materials originally authored by Dr Andrea Krumm at BMG LABTECH HQs.
References
- Lassonade M. et al. (2017). The Challenge of Neurodegenerative Diseases in an Aging Population. https://royalsociety.org/-/media/about-us/international/g-science-statements/2017-may-aging-population.pdf?la=en-GB&hash=C665B04DAB77DE2C053D8F51E61E4379
- EU Joint Programme – Neurodegenerative Disease Research. (2019). Why Choose Neurodegenerative Diseases? https://www.neurodegenerationresearch.eu/about/why/
- Sullivan T. (2019). A Tough Road: Cost to Develop One New Drug is $2.6 Billion; Approval Rate for Drugs Entering Clinical Development is less than 12%. https://www.policymed.com/2014/12/a-tough-road-cost-to-develop-one-new-drug-is-26-billion-approval-rate-for-drugs-entering-clinical-de.html
- Nakamura M. (2012). Real-Time Quaking Induced Conversion Assay for Prion Seeding. BMG Labtech. https://www.bmglabtech.com/real-time-quaking-induced-conversion-assay-for-prion-seeding/
- McGuire et al. (2016) Cerebrospinal fluid real-time quaking-induced conversion is a robust and reliable test for sporadic creutzfeldt-jakob disease: An international study. Ann Neurol. 2016 Jul;80(1):160-5. doi: 10.1002/ana.24679. Epub 2016 Jun 1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4982084/
- Kumar M et al. (2017) Monitoring Early-Stage Protein Aggregation by an Aggregation-Induced Emission Fluorogen. Anal. Chem. 2017, 89, 17, 9322-9329. https://pubs.acs.org/doi/abs/10.1021/acs.analchem.7b02090
- Krasil’nikova et al. 2019. Insulin Protects Cortical Neurons Against Glutamate Excitotoxicity Front. Neurosci., 24 September 2019. https://doi.org/10.3389/fnins.2019.01027
- Ooi J et al. 2015. Inhibition of Excessive Monoamine Oxidase A/B Activity Protects Against Stress-induced Neuronal Death in Huntington Disease. Mol Neurobiol. 2015; 52(3): 1850–1861. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4586002/
- Paliga D et al. (2019). Lethal Factor Domain-Mediated Delivery of Nurr1 Transcription Factor Enhances Tyrosine Hydroxylase Activity and Protects from Neurotoxin-Induced Degeneration of Dopaminergic Cells Mol Neurobiol. 2019; 56(5): 3393–3403. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6476859/
- Ferguson R et al. (2016) PA6 Stromal Cell Co-Culture Enhances SH-SY5Y and VSC4.1 Neuroblastoma Differentiation to Mature Phenotypes. PLoS One. 2016; 11(7): e0159051. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4938384/
- Gyengesi et al. (2019) Chronic Microglial Activation in the GFAP-IL6 Mouse Contributes to Age-Dependent Cerebellar Volume Loss and Impairment in Motor Function. Front. Neurosci., 03 April 2019. https://www.frontiersin.org/articles/10.3389/fnins.2019.00303/full
 About BMG Labtech
About BMG Labtech
BMG LABTECH has been committed to producing microplate readers for more than twenty years. By focusing on the needs of the scientific community, the company’s innovative microplate readers have earned the company the reputation of being a technology leader in the field.
BMG LABTECH has developed a wide range of dedicated and multi-mode microplate readers for life sciences applications and high-throughput screening.
All BMG LABTECH microplate readers are "Made in Germany" and are conceived, developed, assembled, and tested entirely at our headquarters in Germany.
Since our establishment in Offenburg, Germany in 1989, BMG LABTECH has expanded to offer a worldwide sales and support network with offices in the USA, UK, Australia, Japan and France. Our subsidiaries, regional offices and distributors are committed to bringing you innovative microplate reader technology with the quality and reliability you expect from a German company.
Our staff includes engineers and scientists from the fields of biology, biochemistry, analytical chemistry, and physics.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.