ELISA (enzyme-linked immunosorbent assay) was introduced in the 1970s and since then remains a popular approach to measure or validate the presence of an analyte.1,2
In spite of this methods high utility and the continued efforts to improve its sensitivity, washing steps still remain an essential step for most ELISA type assays. Washing steps are either tedious and time-intensive or they require dedicated equipment to complete the task.
Immunoassays using Lumigen’s SPARCL offer no-wash detection. How this luminescent assay can be detected effectively, using the Omega microplate reader has already been demonstrated in an earlier study.3
This article demonstrates the performance of the CLARIOstar® microplate reader in the detection of SPARCL immunoassays, along with the way to improve throughput, demonstrated by performing the SPARCL assay in a 384-well format.
Assay principle
The acronym, SPARCL (spatial proximity analyte reagent capture luminescence), implies that using this approach, an analyte in solution is detected and a light signal is created (Figure 1).
This immunoassay employs two antibodies: one attached to acridan and the other to HRP (horse radish peroxidase). The enzyme and substrate are brought into proximity by the binding of these antibodies to the analyte, and they generate a light signal upon addition of a trigger solution (H2O2). The intensity of the flash of light varies in proportion to the amount of analyte present in the solution, making it a means of measuring the quantity of analyte.
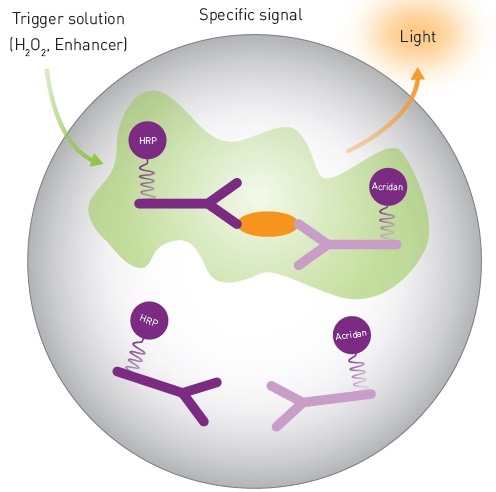
Figure 1. Mechanistic Scheme of SPARCL Technology. Image credits: BMG Labtech.
Materials and methods
- CLARIOstar® microplate reader (BMG LABTECH)
- SPARCL Generic Human IgG Pharmacokinetic Assay Kit (Lumigen)
- White, 384-well plates (Corning)
- White, 96-well plates (Greiner)
1:2 serial dilutions were used to prepare standard curves. Tests using 96-well plates involved preparation of human IgG dilutions in rat serum, with a maximum concentration of 2,875 ng/mL. Human IgG dilution was carried out in PBS with 0.1% BSA to demonstrate the performance of the assay in 384-well plates. The maximum concentration of the solution was 1,000 ng/mL.
Assays using 96 well plates were carried out as outlined in the instructions from Lumigen4, and assays using 384-well plates were carried out as outlined4 but with the following modifications:
- Wells were filled in with antibody mixture (20 µl)
- Then followed with 10 µl of different concentrations of IgG to prepare a standard curve
- After incubation, around 2 µl of background reducing reagent was then added
The CLARIOstar test protocols were used to read the plates with the following instrument settings, which include the addition of a trigger solution.
Instrument settings
|
Detection Mode
|
Luminescence, top reading
|
|
Detection Method
|
Well mode, Kinetic
|
|
No. of intervals
|
100 (96 well), 50 (384 well)
|
|
Interval time(s)
|
0.02
|
|
Gain
|
3500 (96 well), 3300 (384 well)
|
|
Focal height (mm)
|
11.0
|
|
Emission filter
|
No filter
|
|
Injection needle
|
H3
|
|
Injection start time
|
0 seconds
|
|
Injection volume (µl)
|
75 (96 well), 30 (384 well)
|
|
Pump speed
|
300 µl/s
|
|
Aperture spoon
|
For 384 well only
|
Results and discussion
The flash luminescent signal produced by the SPARCL assay has a fairly reproducible signature. The signal detection in a 96-well plate with the CLARIOstar® microplate reader is in good agreement with previously observed results (Figure 2A).
MARS data analysis software was used to obtain a sum of luminescent signal over the period of data collection for 2 seconds. These results demonstrate that the response is strong enough with signal to blank estimated to be 587 for the high concentration standard.
In addition, regression analysis fits the data to a 4-parameter fit as anticipated (Figure 2B), and with which unknown concentrations can be calculated.
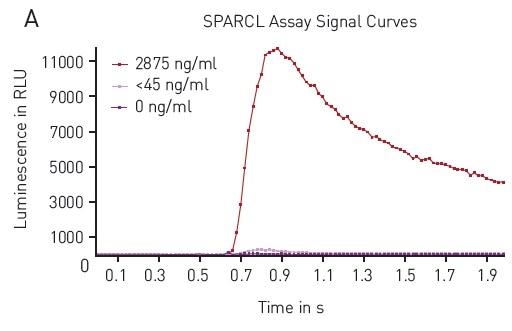
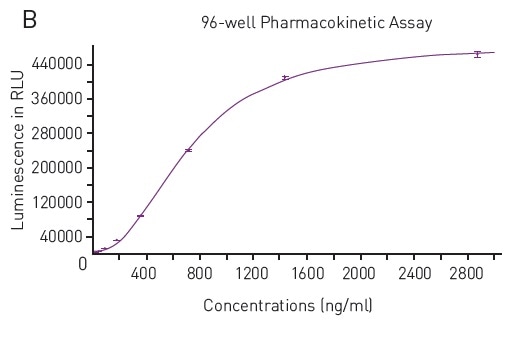
Figure 2. A) Typical Luminescence Output from SPARCL Assay Luminescent signal was capture every 0.02 seconds for 2 seconds and produces these representative curves. Samples depicted contain 2875 ng/mL (red), 45 ng/mL (pink) or 0 ng/mL (purple). B) 4-Parameter Fit Curve from 96-well Assay Data calculated using Sum function corresponds to a 4-parameter fit. R2 value = 0.9995. Image credits: BMG Labtech.
The results also confirmed the ability to carry out the SPARCL assay in a 384-well plate format. The data acquired in the 384-well tests showed a similar response upon addition of the trigger solution (Figure 3A).
Once again, from the data analysis, it is confirmed that the assay carried out in 384-well plates was equally robust with signal to blank values calculated to be 576 for high concentration standard. Also, the concentration can be correlated to signal using the expected 4-parameter fit curve (Figure 3B).
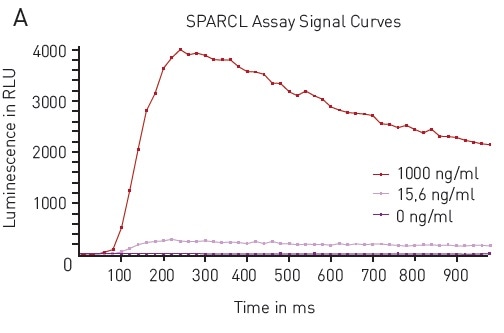
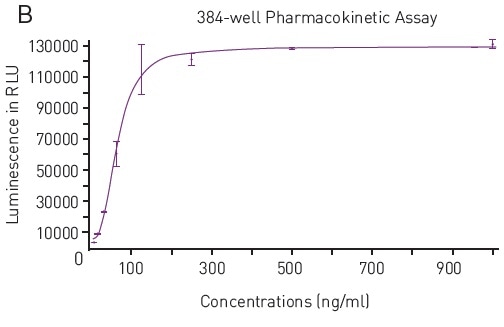
Figure 3. A) Luminescence Output from SPARCL Assay in 384-well microplates luminescent signal was capture every 0.02 seconds for 1 second and produces these representative curves. Samples depicted contain 1000 ng/mL (red), 15.6 ng/mL (pink) or 0 ng/mL (purple). B) 4-Parameter Fit Curve from 96-well Assay Data calculated using Sum function corresponds to a 4-parameter fit. R2 value = 0.9969. Image credits: BMG Labtech.
Conclusion
The results discussed in this article have confirmed the high compatibility of Lumigen’s SPARCL assay with the BMG LABTECH CLARIOstar microplate reader.
Robust responses were obtained in this pharmacokinetic assay used for detecting human IgG. The results have also performed well with the 384-well plate option and can deliver for users looking for higher throughput.
Acknowledgments
Produced from materials originally authored by Mark Cameron1 and Carl Peters2 from:
1 LUMIGEN, Southfield, MI, USA
2 BMG LABTECH Inc., NC, USA
References
- Engvall, E. and Perlman, P. (1971). Enzyme-linked immunosorbent assay (ELISA) quantitative assay of immunoglobulin G. Immunochemistry 8, 871–874.
- Van Weeman, B.K. and Schuurs, A.W.H.M. (1971). Immunoassay using antigen-enzyme conjugates. FEBS Letters 15, 232–236.
- Xie, W., Cameron, M. and Peters, C. (2015). Using SPARCL technology to Develop Immunoassays for Biomarker Detection and Pharmacokinetic Studies.
- For more information about SPARCL contact Lumigen at [email protected]
About BMG Labtech

BMG LABTECH has been committed to producing microplate readers for more than twenty years. By focusing on the needs of the scientific community, the company’s innovative microplate readers have earned the company the reputation of being a technology leader in the field.
BMG LABTECH has developed a wide range of dedicated and multi-mode microplate readers for life sciences applications and high-throughput screening.
All BMG LABTECH microplate readers are "Made in Germany" and are conceived, developed, assembled, and tested entirely at our headquarters in Germany.
Since our establishment in Offenburg, Germany in 1989, BMG LABTECH has expanded to offer a worldwide sales and support network with offices in the USA, UK, Australia, Japan and France. Our subsidiaries, regional offices and distributors are committed to bringing you innovative microplate reader technology with the quality and reliability you expect from a German company.
Our staff includes engineers and scientists from the fields of biology, biochemistry, analytical chemistry, and physics.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.