It has been over a century since altered tumor cell metabolism was first observed. Since that time, metabolic reprogramming has become widely recognized as a core characteristic of cancer, and there have been extensive investigations into the means by which it supports the increased biosynthetic, energetic and redox demands commonly linked to tumor development.
Studies into a range of tumor types have shown that dependence on glutamine and glucose are among the most frequent metabolic requirements of cancer cells. These can both be utilized to fuel ATP production, which is an essential molecule for energy transfer and storage at the cellular level.
Researchers are actively exploring ways of exploiting these and other metabolic vulnerabilities, with a view to embedding their findings into therapeutic purposes.
Traditional approaches in the study of perturbations of ATP and other metabolites are reliant on endpoint assays that can only provide partial kinetic information while lacking cell-specific data in co-culture models. Furthermore, these assays tend to be destructive, effectively blocking any qualitative visual assessment of morphological changes potentially linked to metabolic perturbations.
Nutrient exchange between tumor and stromal cells may mediate resistance to metabolically-targeted treatments, meaning that technologies enabling the direct monitoring of metabolic readouts in co-culture models are vital in the advancement of these types of therapeutics.
Live-cell analysis is a promising approach, offering improved physiological relevance and enhanced insight via the provision of suitable tools to examine cell-specific metabolic readouts and morphology in mono- or co-culture over time with no need for repeated end point measurements.
This article explores the development and potential application of the Incucyte® ATP Assay, incorporating purpose-built instrumentation, novel reagents and robust analytical tools designed with these needs in mind.
Assay principle
The Incucyte® CytoATP Lentivirus Kit has been specially developed to facilitate non-perturbing measurement of dynamic changes in cytoplasmic ATP. The kit includes encoding ATP-binding (CytoATP Lentivirus) and ATP nonbinding (CytoATP Non-binding Control Lentivirus) indicators, as well as a set of two live-cell reagents.
The single cassette, dual fluorescent reporters are made up of cpmEGFP and mKOκ that have been tethered by an ATP binding domain. These are identical other than the fact that the Non-binding Control includes two mutations that prevent ATP binding.
The inclusion of the CytoATP Non-binding Control in live-cell assays allows for calculation of the Corrected ATP Ratio, therefore setting a limit of detection and minimizing the impact of experimental artifacts that could potentially affect fluorescence output (e.g., pH).
Figure 1A outlines the operation of the CytoATP reagent. A conformational change takes place upon ATP binding, bringing the two fluorescent proteins closer together, resulting in an increase in Förster resonance energy transfer (FRET) efficiency from donor (cpmEGFP) to acceptor (mKOκ).
The Incucyte® SX5 Metabolism Optical Module is able to detect these conformational changes by employing a dual excitation and fixed single emission acquisition strategy.
Any ATP-dependent changes in fluorescence are included in the 485X | 578M readout, while reporter expression is exhibited in the direct excitation and emission of mKOκ (535X | 578M). The Incucyte® ATP Analysis Software Module is purpose-built, allowing it to efficiently calculate the images’ ratio of fluorescence intensity (485X | 578M) / (535X | 578M), thus enabling direct measurement of ATP independent of cell number or indicator expression levels (Figure 1A).
The fluorescence emission ratio of the purified protein was measured by utilizing a plate reader. This was done in order to characterize the binding of CytoATP to ATP.
A linear increase in CytoATP’s fluorescence emission ratio was observed across a physiological range of ATP3 (Kd = 2.2 mM at 37° C). Meanwhile, the output of purified Non-binding Control protein was not affected by the presence of up to 40 mM ATP (Figure 1B).
Without the presence of ATP, the ratiometric fluorescence signal for both indicators was found to overlap across a range of pH. Each reporter demonstrated a stable fluorescence ratio that was within the cytosolic physiological range of pH. A decrease in ratio was noted at lower pH values, with both indicators showing identical changes across the full range of values (Figure 1C).
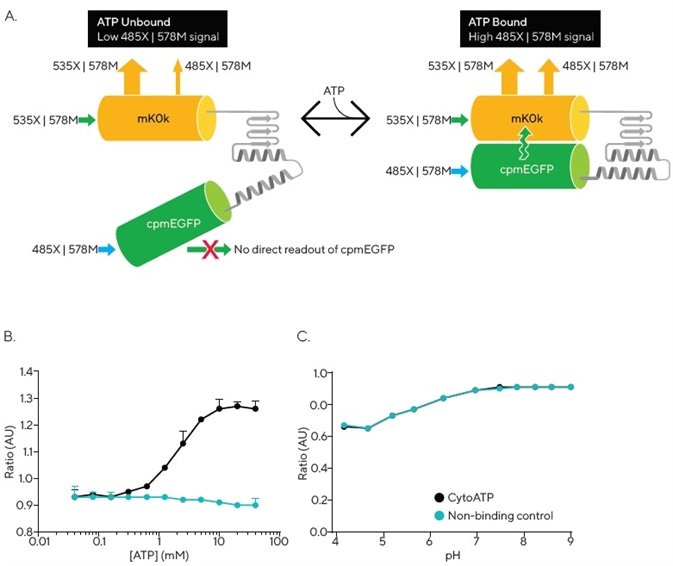
Figure 1. Incucyte® CytoATP and Non-binding Control Lentivirus Reagents. The schematic depicts a conformational change that occurs upon ATP binding, resulting in FRET that can be detected by the Incucyte® SX5 Metabolism Optical Module. Images are acquired using a dual excitation, fixed emission paradigm. The ratio of fluorescence intensity of those images (485X | 578M) / (535X | 578M) is used to evaluate dynamic population changes in ATP (A). In vitro measurements of purified proteins at 37 °C demonstrate ATP dependence of CytoATP and ATP independence of Non-binding Control fluorescence readouts (B). Both indicators show stable ratios near physiological pH and track together across lower pH values (C). Image Credit: Sartorius
Materials and methods
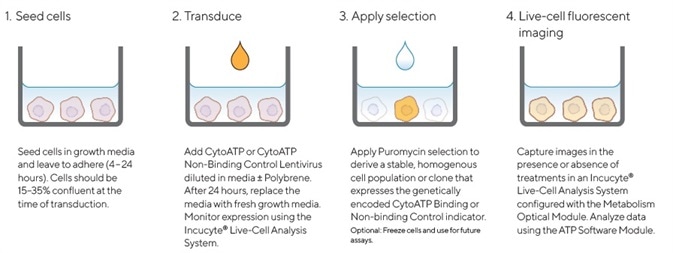
Figure 2. Quick guide for generating cell lines stably expressing Incucyte® CytoATP or Non-binding Control indicators. Image Credit: Sartorius
A CytoATP Lentivirus Reagent Kit was used to generate cell lines that stably expressed CytoATP and Non-binding Control indicators. Multiplicity of Infection (MOI) was then optimized for each cell line via the Incucyte® SX5 Live-Cell Analysis System in order to monitor cell health and expression.
Cells were permitted to recover and achieve suitable expression levels before puromycin selection was applied and non-expressing cells removed. Morphology and growth curves of stably transduced cell lines were found to be comparable with their corresponding parental cell lines across. This was the case across all cell types evaluated (data not shown).
Every live-cell ATP experiment was undertaken with an Incucyte® SX5 that had been configured with an Incucyte® Metabolism Optical Module. All images were obtained and analyzed with the Incucyte® ATP Analysis Software Module.
CellTiter-Glo® ATP assay
A549 cells found to be stably expressing CytoATP indicators were plated at 4000 cells/well or 8000 cells/well in a 96-well microplate. Following overnight incubation, cells were treated with compounds and then monitored in the Incucyte® ATP assay outlined below.
After a two hour compound treatment, this plate of cells was employed to perform a CellTiter-Glo® (CTG) ATP assay (Promega). This assay was performed according to manufacturer instructions, while CTG luminescence measurements were undertaken by utilizing a FlexStation II plate reader (Molecular Devices).
Protein characterization
The fluorescence emission of Non-binding Control and CytoATP purified proteins (0.5 μM final concentration) was measured at a fixed emission wavelength of 590 nm, excited at 488 nm or 551 nm. Indicator response was then calculated as a ratio of the fluorescence emission measured at ex = 488 nm to that measured at ex = 551 nm.
Each measurement was done at 37 °C in a 96-well plate using a Spark plate reader (Tecan). Measurements were done in PBS with varying concentrations of Mg-ATP (equimolar mix of ATP and MgCl2) in order to evaluate ATP-dependence. Furthermore, measurements were performed in buffers in the absence of ATP and with varied pH in order to evaluate pH dependence.
About Sartorius

Sartorius is a leading international pharmaceutical and laboratory equipment supplier. With our innovative products and services, we are helping our customers across the entire globe to implement their complex and quality-critical biomanufacturing and laboratory processes reliably and economically.
The Group companies are united under the roof of Sartorius AG, which is listed on the Frankfurt Stock Exchange and holds the majority stake in Sartorius Stedim Biotech S.A. Quoted on the Paris Stock Exchange, this subgroup is comprised mainly of the Bioprocess Solutions Division.
Innovative technologies enable medical progress
A growing number of medications are biopharmaceuticals. These are produced using living cells in complex, lengthy and expensive procedures. The Bioprocess Solutions Division provides the essential products and technologies to accomplish this.
In fact, Sartorius has been pioneering and setting the standards for single-use products that are currently used throughout all biopharmaceutical manufacturing processes.
Making lab life easier
Lab work is complex and demanding: Despite repetitive analytical routines, lab staff must perform each step in a highly concentrated and careful way for accurate results.
The Lab Products and Services Division helps lab personnel excel because its products, such as laboratory balances, pipettes and lab consumables, minimize human error, simplify workflows and reduce physical workloads
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.