Advanced 3D spheroid models are being widely acknowledged as a vital tool for better evaluation of cancer immunotherapy agents in-vitro. This article describes the potential to kinetically visualize and evaluate immune cell-mediated cytotoxicity of 3D tumor spheroids in co-culture.
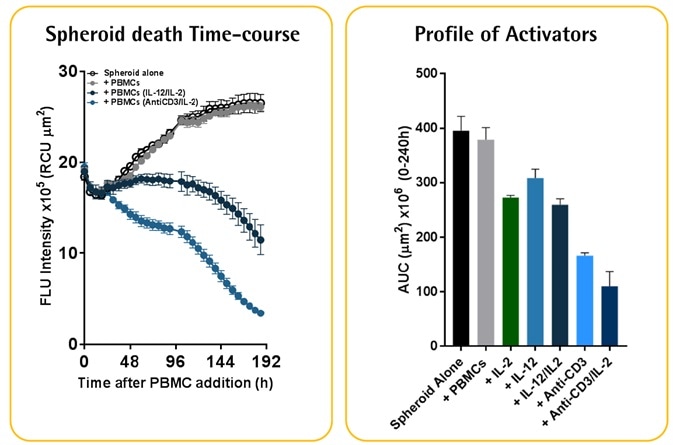
Spheroids were formed in ULA U-bottom plates by using RFP expressing tumor cells. Then, immune cells (PBMCs) were added to quantify the tumor spheroid viability by measuring the loss of RFP fluorescence over time. This technique has been exemplified and verified with several immune cell activators, such as IL-2 and anti-CD3 mediated 3D tumor cell annihilation.
In an ADCC model, Herceptin was found to cause a concentration-dependent cytotoxicity. In contrast to their 2D equivalents, 3D tumor spheroids exhibited improved resistance to Herceptin. These validation techniques exhibit the potential to kinetically image and measure immune cell-induced toxicity within target cancer spheroids in real-time.
Moreover, these assays enable the analysis of advanced tumor spheroid models like CAR-T mediated cytotoxicity.
Effector-to-target ratio-dependent cytotoxicity
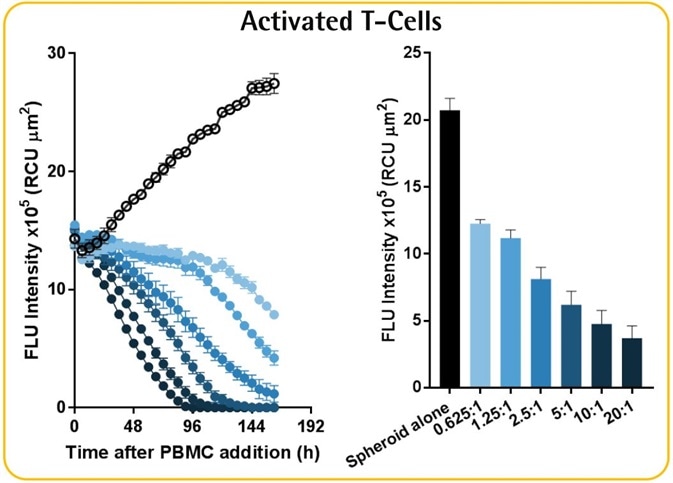
Image Credit: Incucyte®.
Image Credit: Incucyte®
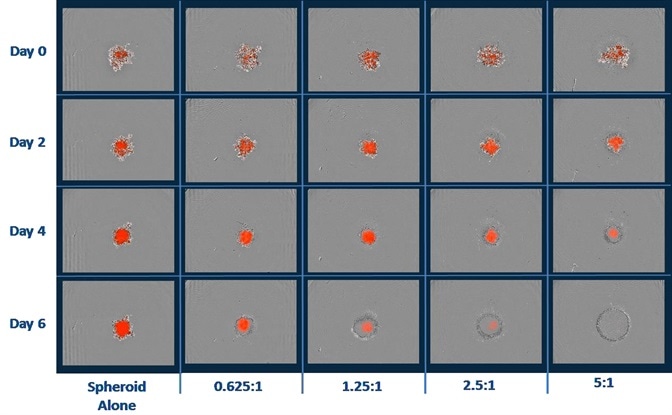
Image Credit: Incucyte®
- Shown above are the blended phase and fluorescent images of A549 NucLight Red™ spheroids when immune cells were present and absent.
- A549 cells (2.5K/well) were seeded with PBMCs that were activated with IL-2 (10 ng/ml) and anti-CD3 (10 ng/ml). Quantification of cytotoxicity was performed based on the red fluorescent intensity.
- Data reveals the annihilation of tumor spheroids by the activated T-cell population, which was dependent on the E:T ratio.
- It must be noted that optimization of E:T ratio is essential as non-targeted cell death was noticed at E:T ratios greater than 5:1.
Herceptin-induced ADCC in HER2-positive SKOV-3 cells
- PBMCs (6.25K/well) were used to seed HER2-negative A549 or HER2-positive SKOV-3 NucLight Red™ spheroids (2.5K/well), which were then treated with Herceptin (mAb-targeting HER2 receptors).
- A Herceptin concentration of more than 10 pg ml−1 induced concentration-dependent blocking of the growth of SKOV-3 spheroids.
- Herceptin-induced cytotoxicity was quantified only in the SKOV-3 spheroids and not the A549 spheroids.
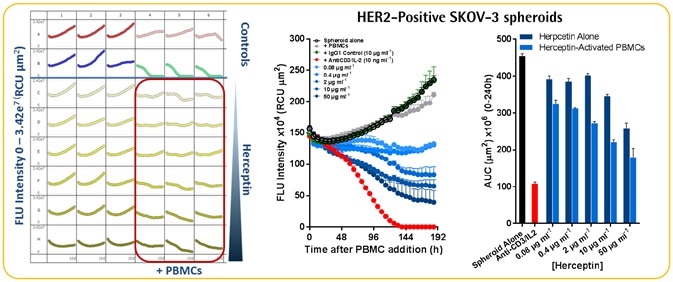
Image Credit: Incucyte®
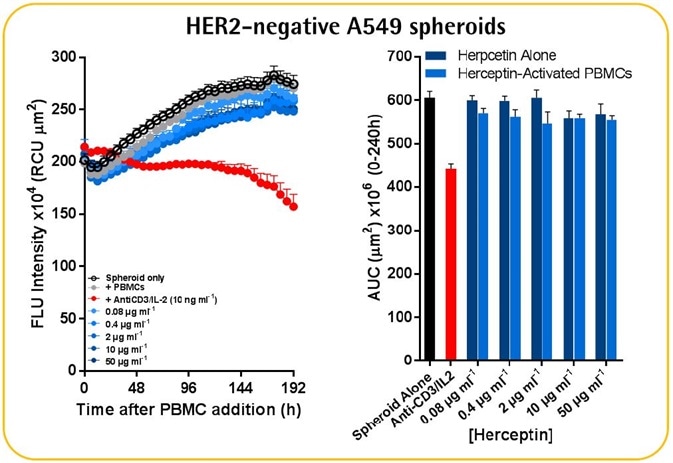
Image Credit: Incucyte®
- A comparable assay was performed in a 2D culture model. SKOV-3 cells (l.6K/well) were seeded overnight before adding the PBMCs (8K/well), and then treated with Herceptin.
- It was found that SKOV-3 tumor spheroids exhibit ~300-fold lower Herceptin sensitivity than the 2D culture model.
- It must be noted that a 34% blocking of the 3D spheroid was apparent at the lowest test concentration of 0.08 pg ml−1. This might indicate the possible existence of a biphasic concentration response curve in which the outermost cells act similar to those in the 2D culture model, whereas the spheroid center exhibits a lower sensitivity.
- Further experimentation is necessary to gain further insights into the differential effects of Herceptin in 2D versus 3D culture models.
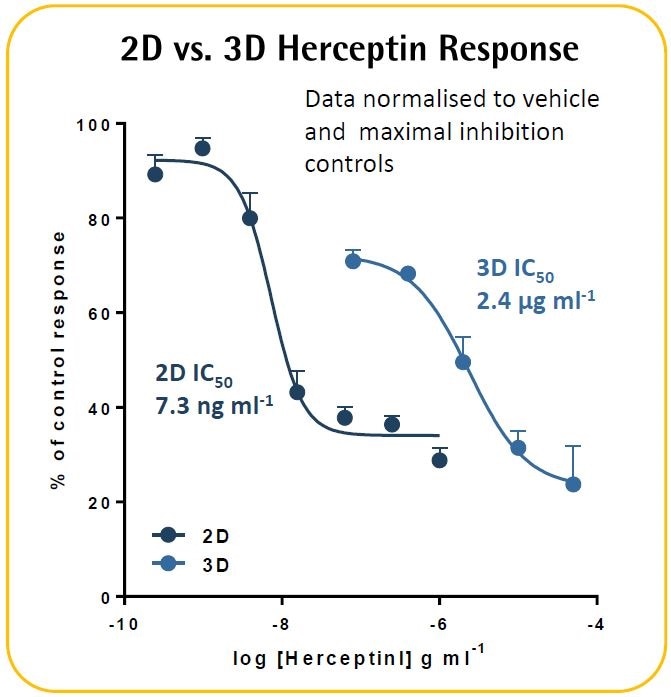
Image Credit: Incucyte®.
Continuous live-cell analysis: methodology

Image Credit: Incucyte®
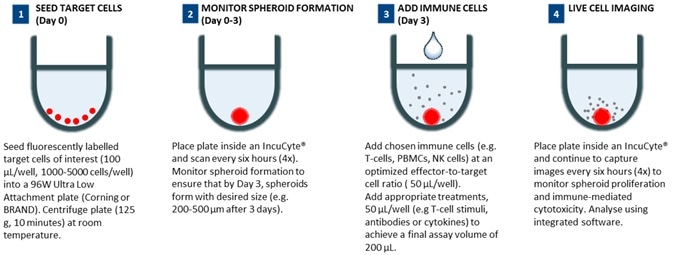
Image Credit: Incucyte®
96-well 3D immune cell killing assay workflow
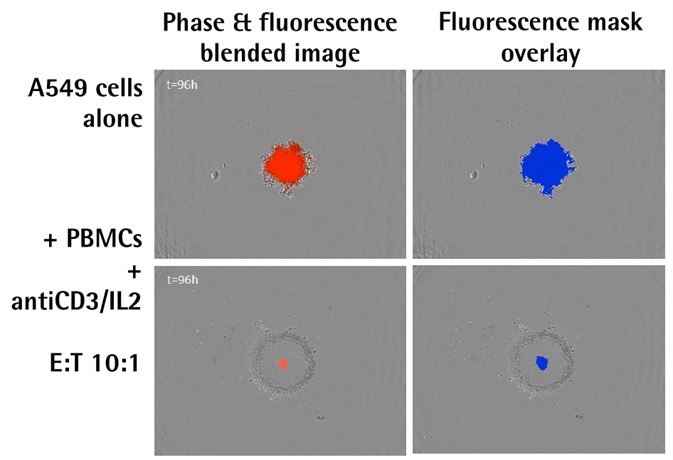
Image Credit: Incucyte®
- The images above illustrate the blended phase and fluorescent images, including the corresponding masks, of A549 human lung epithelial carcinoma cells that stably expressed nuclear restricted RFP (A549 NucLight Red™, Essen Bioscience).
- There is an evident increase in the fluorescence intensity and size of the spheroid alone; however, there is a decline and shrinkage of fluorescence when immune cells are present.
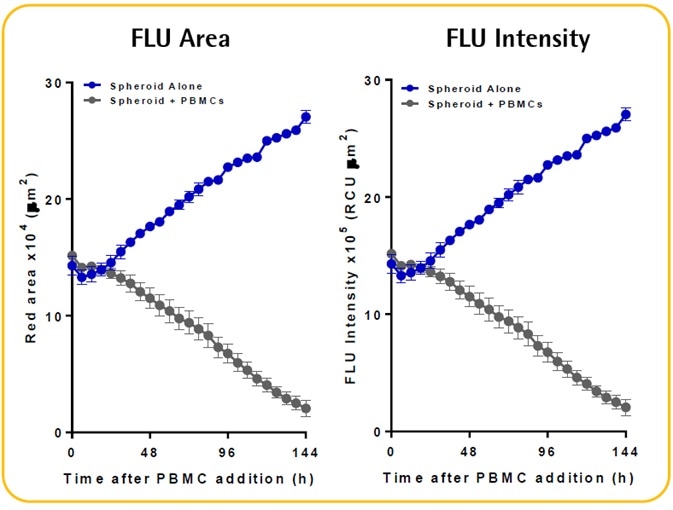
Image Credit: Incucyte®
- The Incucyte® size metrics (fluorescence intensity and fluorescence area) can be used to kinetically measure the immune cell-mediated cytotoxicity and spheroid proliferation. These metrics necessitate the fluorescent spheroid to be masked.
Activator-dependent tumor cytotoxicity
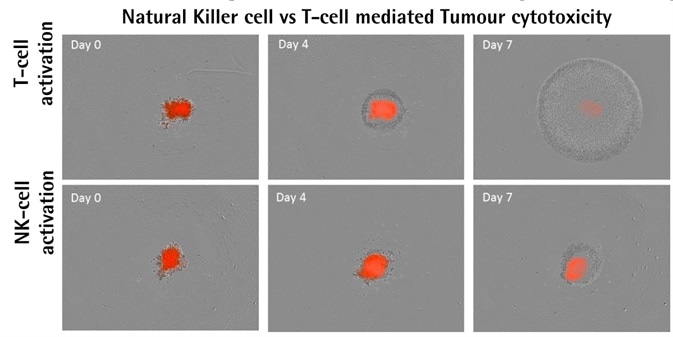
Image Credit: Incucyte®
A549 NucLight Red™ cells (2.5K/well). Optimized E:T ratios of 2.5:1. Image Credit: Incucyte®
- Isolated PBMCs activated with IL-12/IL2 (10 ng ml−1) or anti-CD3/IL-2 (10 ng ml−1), which respectively target NK-cell or T-cell populations, were used to treat A549 NucLight Red™ cells.
- The result of activating different populations of PBMCs was the differential impacts on the destruction of tumor spheroids.
- When compared to activated NK-cells, populations activated by T-cells were found to proliferate more.
- Spheroid destruction mediated by T-cells occurred faster than that mediated by NK-cells.
- Data shows that spheroid destruction mediated by T-cells is induced more by the existence of anti-CD3 antibody.
- The frequencies of cell types in PBMC populations differ from one donor to another. In general, 4570% of PBMCs are CD3+ T-cells, but only 520% of PBMCs are NK cells. This inconsistency in occurrence could explain the differential effects found in the cytotoxicity of tumor spheroids.
Acknowledgments
Produced from materials originally authored by M. Oliver, K. Patel, N. Holtz, E. Endsley, A. Overland, T. Dale and D. Trezise from Essen BioScience.
About Incucyte®

The Incucyte® Real-Time Quantitative Live Cell Analysis System, designed by Essen BioScience, Inc (now a Sartorius company) is the first system to continuously quantify cell behavior over time (from hours to weeks) while cells remain undisturbed inside a standard incubator. The Incucyte® System automatically collects and analyzes images continuously around the clock, providing insight into active biological processes that is difficult to achieve with endpoint assays. The Incucyte® suite of assays utilize proprietary reagents that do not perturb cell health with validated 96/384 well format protocols that save time and money.
Incucyte® Live-Cell Analysis has revolutionized numerous studies, with applications such as stem cell monitoring and reprogramming, label-free analysis, ATP metabolism, multiplexing, neurite outgrowth and dynamics, migration/invasion, 3D-spheroids, angiogenesis, NETosis, reporter gene expression, immunocytochemistry, immune cell killing, apoptosis, cytotoxicity, kinetic data generation, antibody internalization. All this without the removal of cells from an incubator.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.