Sponsored Content by SartoriusReviewed by Alex SmithJun 30 2022
When experimenting with cell cultures, contaminants in water can come in a number of different types, such as yeasts, molds or bacteria. Typically, these contaminants can be observed using optical microscopy or by the eye.
Contamination from alternative biological agents or chemicals may also impact the morphology, behavior or growth of cultured cells. However, these can go unnoticed during a simple visual analysis.
Therefore, it is important to ensure that the water that is employed in cell cultures is microorganisms-free, particularly from inorganic ions (heavy metals such as zinc, lead, etc.) endotoxins and other organic compounds (pesticides, humic acids, tannins, etc.).
For a detailed overview, please refer to the reference literature.1, 2 Examples of frequently occurring impurities in mains water (tap water) and target values for cell culture work are displayed in Table 1.
Table 1. Typical mains water impurities and target values for cell culture work2. Source: Sartorius
| Parameter |
Mains Water |
Water for Cell Culture |
% Reduction |
| Conductivity (μS/cm) |
50 to 900 |
0.2 |
99.95 |
| Calcium (mg/L) |
20 to 150 |
< 0.01 |
> 99.99 |
| Sodium (mg/L) |
20 to 150 |
< 0.01 |
> 99.99 |
| Iron (mg/L) |
0.01 to 0.1 |
< 0.001 |
> 98 |
| Bicarbonate (mg/L) |
30 to 300 |
< 0.01 |
> 99.99 |
| Chloride (mg/L) |
10 to 150 |
< 0.01 |
> 99.99 |
| Sulphate (mg/L) |
1 to 100 |
< 0.01 |
> 99.98 |
| TOC (mg/L) |
0.2 to 5 |
0.1 |
96 |
| Free chlorine (mg/L) |
0.1 to 0.5 |
< 0.01 |
> 97 |
| Bacteria (CFU/100 mL) |
100 to 1000 |
< 10 |
> 98 |
| Endotoxin (IU/mL) |
1 to 10 |
< 0.1 |
> 98 |
| Turbidity |
0.1 to 2 |
< 0.01 |
> 99 |
The aim of the current series of tests was to assess if ultrapure water produced using the Arium® Pro UF could be used in applications that make use of cell cultures without raising any significant problems.
For the purpose of this study, PER.C6 EpCAM cells were cultured in ready-made CDM4PERMab (Hyclone) media used as controls, as well as in CDM4PERMab (Hyclone) powder media prepared with ultrapure water taken from the Arium® Pro UF (UF water) and RO water, respectively, for test purposes.
For this study, data for RO water was acquired by the predecessor model (Arium® RO) of the current Arium® advanced system. Each culture’s results were subsequently utilized to evaluate if the water from the Arium® Pro UF is appropriate when cultivating PER.C6 EpCAM cells.
Derived from human retinoblast cells, the PER.C6 cell line defined and utilized in this series of tests was also used for the expression of monoclonal antibodies (mAb production) and recombinant proteins, as well as for the production of therapeutic proteins and monoclonal antibodies.
Ultrapure water system
The system (Figure 1) has been developed to produce ultrapure water from pre-treated water sources by eliminating trace levels of residual contaminants. Generating ultrapure water necessitates constant recirculation and continuous flow.

Figure 1. Arium® Pro UF water system. Image Credit: Sartorius
This is executed by a pump system that manages the pressure. The system measures the water’s conductivity at both the water feed inlet and the water outlet (downstream product). The system is able to function using two different cartridge kits.
These cartridges are loaded with a special active carbon adsorber and a special mixed-bed exchange resin designed to supply high-purity water with low extractables. A final microfilter is typically installed at the outlet to eliminate any particles or bacteria from the ultrapure water as it is distributed.
The typical process for water purification is depicted and described in Figure 2.
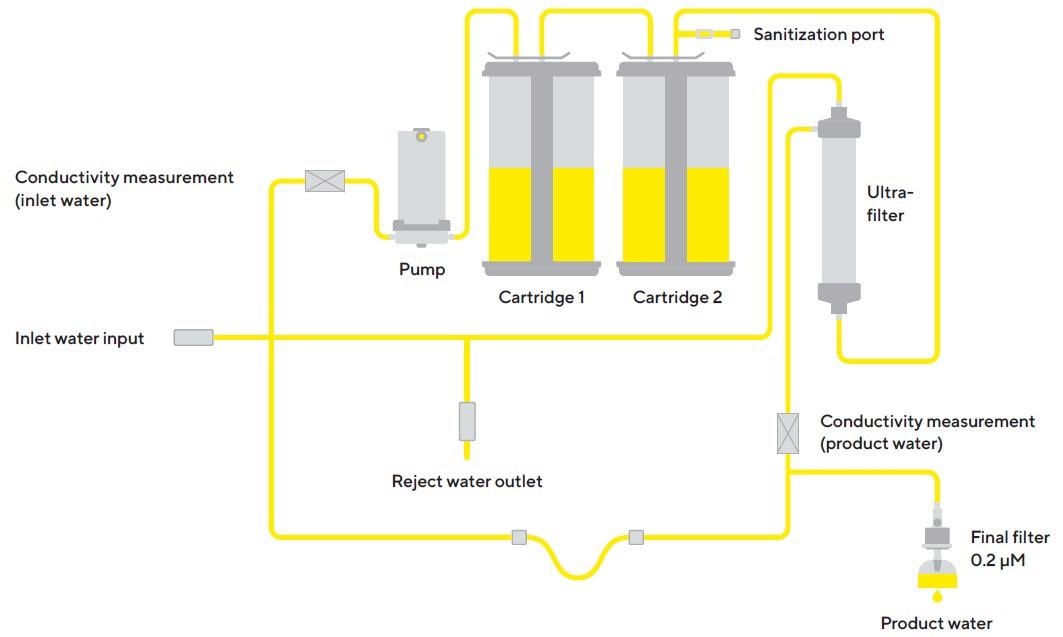
Figure 2. Schematic drawing of the working principle of the Arium® Pro UF ultrapure water system. Image Credit: Sartorius
The feed water for the Arium® Pro UF system (predecessor model with the same technical design as the current Arium® Pro UF device displayed in Figure 1), for the tests mentioned below, was prefiltered by an Arium® RO reverse osmosis system.
This configuration complies with Whitehead2 for the water purification treatment for small-scale cell cultivation in laboratories. This pretreatment system is not specified further in this article.
Methods and materials
Cultivation of PER.C6 EpCAM cells (eye catcher) was conducted using T-75 flasks, with vented caps (Nunc) for gas exchange, in duplicates (12 mL media in each flask) for 10 passages, and in 125 mL spinner flasks (Wheaton, VWR) with 50 mL of media in duplicates.
The PER.C6 EpCAM cell line was cultured in CDM4PERMab ready-made media (Hyclone) and in CDM4PERMab powder media (Hyclone).
Reconstitution of the CDM4PERMab powder media was carried out using RO water or UF water, as well as 4 mM L-glutamine (Lonza), sodium bicarbonate (3.2 g/L, Merck) and pluronic acid F-68 (0.5 g/L, Sigma), and then, under aseptic conditions, passed through a final 0.2 µm sterilizing-grade filter using 1000 mL disposable filtration units (Sartolab®, Sartorius).
The seeding density of the cells was 0.3 x 106 cells/mL in T-75 flasks and 0.7 x 106 cells/mL in spinner flasks. A CO2 incubator (Forma direct heat CO2 incubator, Model 3-11 Thermo Scientific) was used to incubate the T-flasks and spinner flasks at 37 °C, 5% CO2 and 85% humidity.
In the CO2 incubator, a magnetic stirrer (VWR) was used to incubate the spinner flasks at 80 rpm, with the sidearm of each spinner flask capped loosely to allow gas exchanges inside. Samples were extracted every weekday (except weekends/Days 4 and 5) from the spinner flasks and every third day from the T-flasks to establish the viable cell density.
The viable cell density was recorded in accordance with the Trypan Blue exclusion method utilizing a hemocytometer (Vasa Scientific). Full detailed information about the fundamental techniques of cell cultivation is offered in the reference literature.3
Results and discussion
The mean cell density yield in the control T-flasks (cells cultured in ready-made media as controls) was 1.52 x 106 cells/mL, and the average feasibility for these controls was 95.23% (Figure 3A).
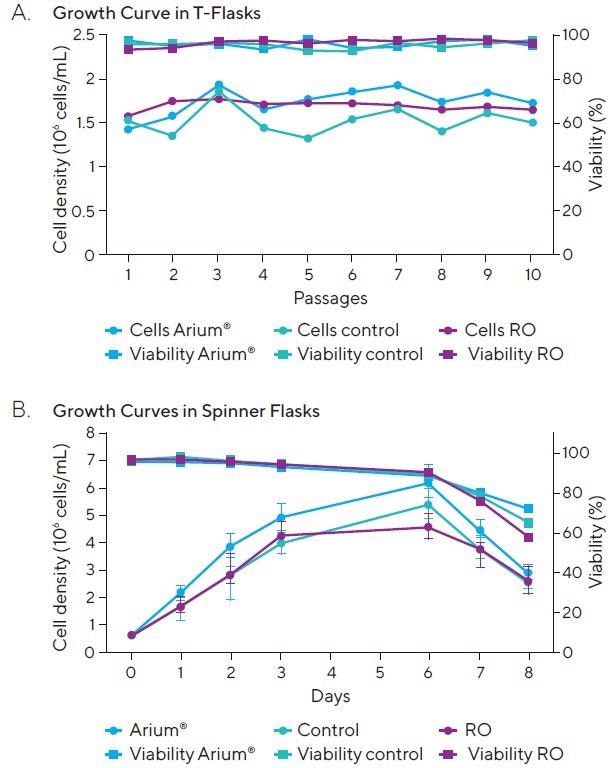
Figure 3. (A) Growth curves of PER.C6 EpCAM cell lines in T-flasks. (B) Growth curves of PER.C6 EpCAM cell lines in spinner flasks. Image Credit: Sartorius
For cells in T-flasks cultured in UF water-reconstituted media, the mean cell density was 1.73 x 106 cells/mL, and an average viability of 95.7% was achieved. In comparison with these results, a mean cell density yield of 1.68 x 106 cells/mL and a viability of 95.59% (Figure 3A) were achieved in the cells cultured in media reconstituted in RO water.
In an additional experiment, the PER.C6 EpCAM cell lines were cultured in spinner flasks with ready-made media (control), media reconstituted using UF water and media reconstituted using RO water (Figure 3B).
The maximum cell density achieved in control spinner flasks was 5.42 x 106 cells/mL with an 88.47% feasibility, 6.24 x 106 cells/mL with an 88.55% feasibility in UF water spinner flasks and 4.60 x 106 cells/mL with an 89.85% feasibility in RO water spinner flasks on Day 6 of cultivation (Figure 3B).
Microscopic examination of the cells in the RO spinner flasks revealed that cells cultured in media reconstituted with RO water appeared in ill-health compared with cells grown in the ready-made media and in media reconstituted with UF water.
The viability of the cells grown in spinner flasks with media reconstituted in RO water decreased more quickly than those cultured in the ready-made media and media reconstituted with UF water.
This decrease was not noted when the cells were cultured in T-flasks. The quick decline in feasibility inside the spinner flasks can be associated with the presence of endotoxins and inorganic salts in RO water which influence the growth and viability of the cells.
However, these severe effects of endotoxins or inorganic salts were not seen in static cultures (small-scale cultivation), such as in the T-flasks, because in the latter case, growth is restricted by the O2 concentration in the medium and not by the endotoxin or inorganic salt concentration (no conventional growth curve was noted in the T-flasks compared with the cultures in the spinner flasks).
The actual effects can only be observed in spinner flask cultivation, where higher cell densities emerge and O2 is not a restricting factor.
In spinner flasks where greater cell densities can be achieved, the effect of higher concentrations of endotoxins and inorganic salts in media reconstituted with RO water result in a restricted growth rate (lower cell density and lower viability) in comparison to values acquired for the controls (ready-to-use media) or for the samples in medium reconstituted with UF water.
These results are affirmed by the antibody production (mAb) experiments. MAb production in spinner flasks (Figure 4) of cells cultured in media reconstituted with UF water was 0.84 mg/mL (example Day 8) and is, therefore, greater than the values acquired for mAb production with the controls being the manufacturer’s ready-made media (0.71 mg/mL) or in the samples reconstituted with RO water (0.42 mg/mL).
The productivity of cells, i.e., mAb production in T-flasks, was not taken because the antibody numbers were too low and, as such, an appropriate comparison of such low values was not statistically possible.
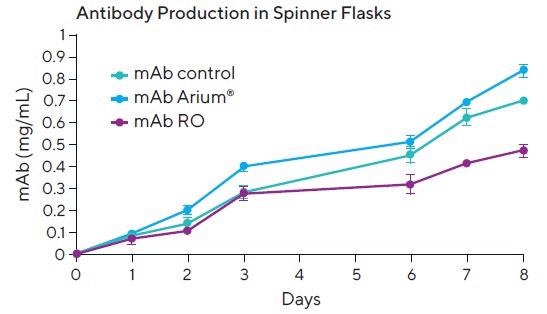
Figure 4. Antibody production in cells cultivated in media reconstituted with UF water (mAb Arium®), ready-made media (mAb Control) and RO water (mAb RO) in spinner flasks. Image Credit: Sartorius
Conclusions
The above results clearly indicate that dehydrated media (CDM4PERMab media) reconstituted with UF water are viable for the cultivation of PER.C6 EpCAM cell lines in place of commercially available ready-to-use media.
The growth features of the PER.C6 EpCAM cell lines cultured in media reconstituted with UF water are close to those of the PER.C6 EpCAM cell lines cultured in pre-prepared CDM4PERMab media used as controls.
Moreover, it was noted that the growth in cell line samples was enhanced in those cultured in media reconstituted with UF water in contrast to the samples cultured in media reorganized with RO water when the experiments were performed in spinner flasks.
Here, greater cell densities typically occur, and O2 is not a restricting factor. Therefore, it can be concluded that the greater concentration of endotoxins or inorganic salts in RO water was responsible for the decrease in growth.
These results were validated and reflected by the mAb production in the PER.C6 EpCAM cell line cultured in spinner flasks. MAb production from PER.C6 cell lines exhibited the most significant amounts in the samples reconstituted with Arium® Pro UF ultrapure water, subsequently followed by the controls (ready-to-use media).
These findings were distinct from those in the RO water samples, where mAb production declined.
Therefore, it was concluded the Arium® Pro UF system water is, in fact, well-suited for PER.C6 EpCAM cell cultivation because this water system significantly reduces the impurities, including organic compounds and inorganic ions, and, specifically, it decreases endotoxins, as confirmed in more recent experiments.4
References
- ASTM Standard Guide for Bio-Applications Grade Water D 5196-06 (2018). https://www.astm.org/Standards/ D5196.html
- Whitehead, P. (2007). Water Purity and Regulations. Stacey, G.N., & Davis, J. (Eds.) Medicines from Animal Cell Culture. John Wiley & Sons, Ltd.
- Freshney, I.R. (2010). Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications, Sixth edition. John Wiley & Sons, Inc.
- Schmidt, K. & Herbig, E. (2012). Weniger ist mehr— Quantitative Endotoxinbestimmung von Reinstwasser. Laborpraxis, 5, 36. Jhg.
About Sartorius

Sartorius is a leading international pharmaceutical and laboratory equipment supplier. With our innovative products and services, we are helping our customers across the entire globe to implement their complex and quality-critical biomanufacturing and laboratory processes reliably and economically.
The Group companies are united under the roof of Sartorius AG, which is listed on the Frankfurt Stock Exchange and holds the majority stake in Sartorius Stedim Biotech S.A. Quoted on the Paris Stock Exchange, this subgroup is comprised mainly of the Bioprocess Solutions Division.
Innovative technologies enable medical progress
A growing number of medications are biopharmaceuticals. These are produced using living cells in complex, lengthy and expensive procedures. The Bioprocess Solutions Division provides the essential products and technologies to accomplish this.
In fact, Sartorius has been pioneering and setting the standards for single-use products that are currently used throughout all biopharmaceutical manufacturing processes.
Making lab life easier
Lab work is complex and demanding: Despite repetitive analytical routines, lab staff must perform each step in a highly concentrated and careful way for accurate results.
The Lab Products and Services Division helps lab personnel excel because its products, such as laboratory balances, pipettes and lab consumables, minimize human error, simplify workflows and reduce physical workloads.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.