Sponsored Content by SartoriusReviewed by Alex SmithApr 28 2022
T cells are produced and released from the thymus as naive T cells (TN). Following infection or antigen exposure, TN cells proliferate and differentiate into effector T cells (TE), which travel to infected target cells and destroy them, thus clearing the infection.
After the infection has been addressed, the majority of the TE die, but a small percentage of them develop into long-lived resting-state memory T cells (TM). These TM cells accumulate over time and can provide lifelong protection against a variety of diseases and pathogens.1
T naive cells (TN), T central memory cells (TCM), T stem cell-like memory cells (TSCM), T effector memory cells (TEM), T transitional memory cells (TTM), T effector memory cell re-expressing CD45RA (TEMRA), and T terminal effector (TTE) cells are all identified by the positive or negative expression of certain surface markers such as CD45RO, CD45RA, CD62L, CD27 and CD95.
T cells’ effector function increases as they transition from TN to memory and effector cells, while their ability to self-renew (proliferate) decreases.2
In patients with relapsed/refractory cancer, adoptive therapy with ex vivo expanded antigen-specific T cells, with or without genetic modification, can stimulate remissions. The therapy’s success depends on the effective expansion of T cells ex vivo and their persistence, homing and cytotoxicity after administration to the patient.3
T cells from cancer patients are more differentiated and difficult to expand than T cells from healthy donors. The biodistribution and long-term persistence of TSCM and TCM are attractive targets for overcoming the current limitations of cancer adoptive therapy.
Ex vivo expansion protocols are being studied in the hopes of increasing the clinical efficiency of genetically modified cells used in adoptive cell therapy by generating and maintaining less differentiated subset phenotypes.4,5,6
One key challenge is that a patient’s T cells may be permanently damaged as a result of disease or prior treatment. Due to the absence of ex vivo expansion potential, not all patients with the disease are candidates for adoptive cell therapy. This suggests that to effectively treat cancers, individualized T cell subset profiling and personalized immunotherapy are required.7
Ex vivo T cell expansion remains a critical step in adoptive T cell therapies such as CAR-T, TCR, and TIL cell therapies. The iQue® Human T Cell Memory Kit was created to characterize and profile ex vivo expanded T cells using high-throughput flow cytometry. This assay’s miniaturized format saves both reagents and valuable samples.
The simultaneous measurement of T cell phenotypes and secreted functional cytokines is enabled by multiplexing cells and beads in each assay well, a workflow that was previously accomplished on two separate instruments.
The assay is designed to work with the iQue® platform, which not only offers quick sample acquisition (5 minutes per 96-well plate) but also includes advanced immune screening and profiling algorithms like profiling maps and multi-plate analysis. These tools, when used together, allow data to be quickly transformed into actionable results.
Human peripheral blood mononuclear cells (PBMCs) from three donors were cultured in a single, 96-well plate for three days under 14 different culture conditions as part of proof-of-concept (POC) studies.
The iQue® Human T Cell Memory Kit can be used to easily determine T cell culture conditions, like media supplemented with cytokines IFNß or IL-21, that could increase the frequency of less differentiated T memory cells like TCM and TSCM.
More intriguingly, secreted IL-10 was found to have a significant correlation with TCM frequency in PBMCs, suggesting that secreted IL-10 could be used as an early indicator of increased TCM frequency in ex vivo T cell expansion culture.
These findings suggest that when combined with the iQue® platform and integrated Forecyt® software, the iQue® Human T Cell Memory Kit could provide a reliable solution for the characterization of T memory cells and ex vivo expanded T cell products for adoptive T cell therapy.
Assay principles
Live immune cells are distinguished from dead cells in each assay well as they are stained with a fluorescent membrane integrity dye. Only dead cells and those with a compromised membrane allow the dye to enter, staining nucleic DNA via intercalation.
Live cells are immunophenotyped using a fluorescent antibody panel to distinguish between CD3+ (T cells), CD3- (non-T cells), CD4+ (T helper cells), and CD8+ (non-T cells) (T cytotoxic cells). CD45RA, CD45RO, CD27, CD62L and CD95 are among the five T cell surface markers included in the panel for phenotyping T naive | memory | effector cells.
Effector cytokines produced by ex vivo expanded T cells, such as pro-inflammatory cytokine IFN and anti-inflammatory cytokine IL-10, are measured using two different iQue® Qbeads® in the same well in a “sandwich” immune assay format (Figure 1).
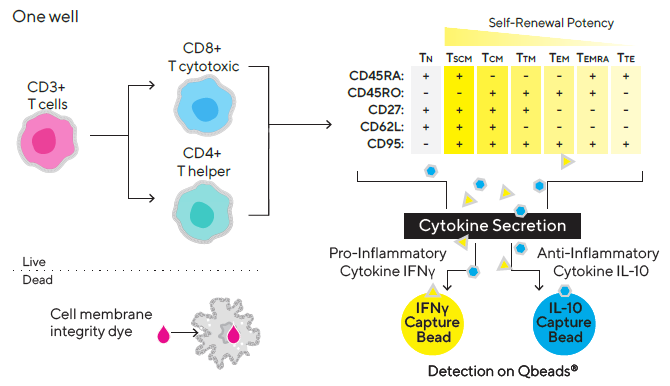
Figure 1. Multiplex assay design. Image Credit: Sartorius
By integrating the iQue® Human T Cell Memory Kit with the iQue® Human T Cell Companion Kits of the user’s choice, up to six additional cytokines can be measured in a multiplexed assay format if necessary (IL-2, IL-6, IL-13, IL-17A, TNF, or GM-CSF).
Materials
Cells and reagents
Cryopreserved human PBMCs (Astarte Biologicals) were cultured in Lonza’s X-VIVOTM 15 media, which included 5% human AB sera (Sigma). T cells were activated and expanded ex vivo using CD3/CD28 Dynabeads® (ThermoFisher Scientific) and cytokines IL-4, IL-6, IL-7, IL-15, IL-21, and IFNß (PeproTech).
The iQue® Human T Cell Memory Kit was utilized to determine cell count, viability, and secreted cytokines (INFγ and IL-10), and to distinguish memory T cell subsets based on cell surface phenotypic markers (CD27, CD45RO, CD45RA, CD62L and CD95).
Methods
Cell treatment
Three healthy donors’ PBMCs were recovered overnight in X-VIVO15 media containing 5% human AB sera (referred to as media hereon). On Day 0, each donor’s cells were counted and the cell density was set to 4 million/mL (4X).
Cells were plated at a density of 25 μL/well into the wells of a 96-well v-bottom plate (Cat. No. 10149). Before use, CD3/CD28 Dynabeads® were washed once with media. The beads and wash media were then placed for a minimum of 1 minute on a DynamagTM magnet (ThermoFisher Scientific).
The beads were then resuspended in fresh media at a density of 0.8 million/mL after the wash media was removed (4X). Dynabeads® (25 μL/well) were added to the assigned wells in the culture plate. Around 14 different cytokine cocktail combinations were prepared at (2X) in media.
These mixtures were added to designated wells at a rate of 50 μL/well. The final cell density was one million cells per mL, and the final CD3/CD28 Dynabeads® density was 0.2 million cells per mL (final cell/bead ratio: 4:1).
The final cytokine concentrations in culture wells were: IL-7 and IL-15, 10 ng/mL; IL-4, IL-6, IL-21, and IFNß, 100 ng/mL. Figure 2 reveals the 14 cytokine combinations (Treatments 3–16). Treatment 1 is negative control (media and cells only) and Treatment 2 is cells only activated with Dynabeads® (no supplemented cytokines).
To enable T cell population expansion with minimal edge effect, the prepared culture plate was kept in a high humidity chamber in a 37 °C incubator with 5% CO2 for up to eight days.
| Treatment |
Cocktail |
| 1 |
Negative Control |
| 2 |
CD3 CD28 Dynabeads® (DB) |
| 3 |
IL-4, Il-7, DB (Core) |
| 4 |
IL-6 |
| 5 |
IL-15 |
| 6 |
IL-21 |
| 7 |
IFNβ |
| 8 |
IL-15, IL-6 |
| 9 |
IL-21, IL-6 |
| 10 |
IFNβ, IL-6 |
| 11 |
IL-21, IL-15 |
| 12 |
IFNβ, IL-15 |
| 13 |
IFNβ, IL-2 |
| 14 |
IL-21, Il-15, IL-6 |
| 15 |
IFNβ, IL-15, IL-6 |
| 16 |
IFNβ, IL-21, IL-15 |
| |
1 |
|
2 |
3 |
|
4 |
5 |
|
6 |
7 |
|
8 |
9 |
|
10 |
11 |
|
12 |
| A |
|
1 |
|
|
9 |
|
|
1 |
|
|
9 |
|
|
1 |
|
|
9 |
|
| B |
|
2 |
|
|
10 |
|
|
2 |
|
|
10 |
|
|
2 |
|
|
10 |
|
| C |
|
3 |
|
|
11 |
|
|
3 |
|
|
11 |
|
|
3 |
|
|
11 |
|
| D |
|
4 |
|
|
12 |
|
|
4 |
|
|
12 |
|
|
4 |
|
|
12 |
|
| E |
|
5 |
|
|
13 |
|
|
5 |
|
|
13 |
|
|
5 |
|
|
13 |
|
| F |
|
6 |
|
|
14 |
|
|
6 |
|
|
14 |
|
|
6 |
|
|
14 |
|
| G |
|
7 |
|
|
15 |
|
|
7 |
|
|
15 |
|
|
7 |
|
|
15 |
|
| H |
|
8 |
|
|
16 |
|
|
8 |
|
|
16 |
|
|
8 |
|
|
16 |
|
| |
|
|
Donor 1 |
|
|
|
Donor 2 |
|
|
|
Donor 3 |
|
|
| |
Possible Effect |
| CD3 | CD28 Dynabeads® |
T cell stimulation | expansion |
| IL-4 | IL-7 |
Help in vitro expansion | maintain memory T cells |
| IL-6 |
Promote T follicular helper cells |
| IL-15 |
Increase survival of memory T cells |
| IL-21 |
Increase Th17 cells and CD8 effector function |
| IFNβ |
Increase CD8 effector function and survival |
Figure 2. Plate design and screening conditions. Image Credit: Sartorius
Assay setup
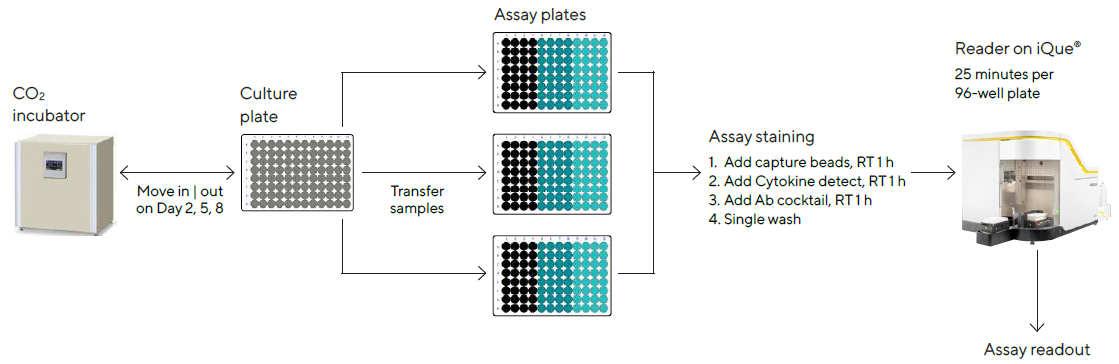
Culture samples were manually mixed by up-and-down pipetting on days 2, 5 and 8 post-treatment to guarantee cell resuspension, and 10 μL of the sample comprising cells and supernatant were passed from the culture plate to an assay plate (Cat. No. 10149).
The iQue® Human T Cell Memory Kit was used to analyze the samples, as shown in Figure 3. The assay took about 3 hours in total, with 30 minutes of hands-on time each day. The iQue® was used to collect data, with a total acquisition time of about 25 minutes per 96-well plate.
| Basic T Cell ID |
T Naive | Memory | Effector ID |
Secreted
Cytokines |
Cell
Count |
Viability |
| CD3+ |
CD4+ |
TN |
TSCM |
TCM |
TTM |
TTEM |
TEMRA |
TTE |
IFNγ |
IL-10 |
+ |
+ | - |
| CD8+ |
TN |
TSCM |
TCM |
TTM |
TTEM |
TEMRA |
TTE |
Figure 3. Screening workflow. Image Credit: Sartorius
The integrated Forecyt® software generated all of the experimental metrics, including T cell identifiers (CD3/CD4/CD8), T cell memory subsets (naïve, memory, effector), cell viability and quantitation of secreted cytokines.
Data from each donor was normalized against samples from the same donor treated with only CD3/CD28 Dynabeads® and expressed as the fold increase using the sophisticated metric tool in Forecyt® to evaluate the effect of the cytokine treatment.
On every assay day, a distinct plate with two rows of cytokine standards was used to create standard curves for cross-plate quantitation of IFN and IL-10 in the screening plate employing Forecyt® using the iQue® Human T Cell Memory Kit.
Data acquisition and analysis
The assay template included with the iQue® Human T Cell Memory Kit was used to collect samples on the iQue®. Due to the kit’s antibody panel design, the violet, blue and red (VBR) laser configuration is required.
Using the iQue® Human T Cell Memory Kit template and Forecyt® software, the acquisition protocol and data analysis, including data metrics, event gates and gating strategy (Figures 4 and 5), heat maps and standard curves, were auto-generated.
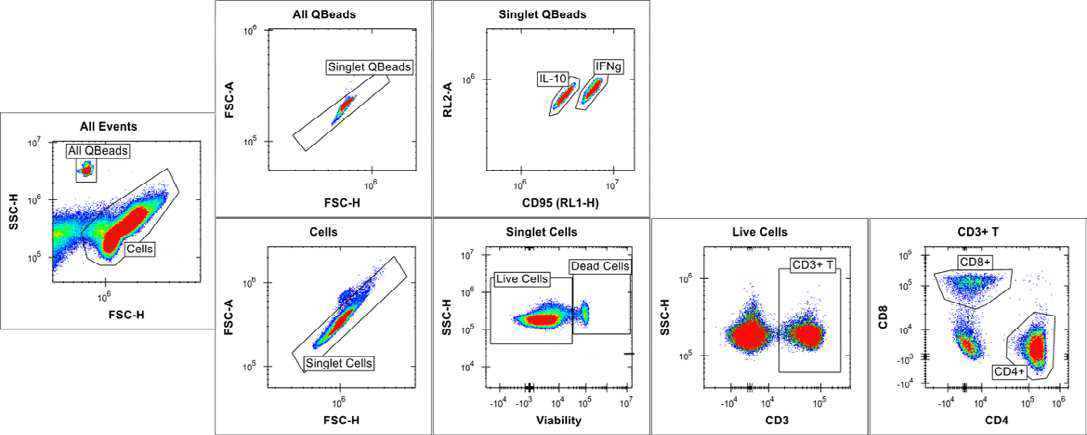
Figure 4. Cell and bead gating strategy at full plate level. Image Credit: Sartorius
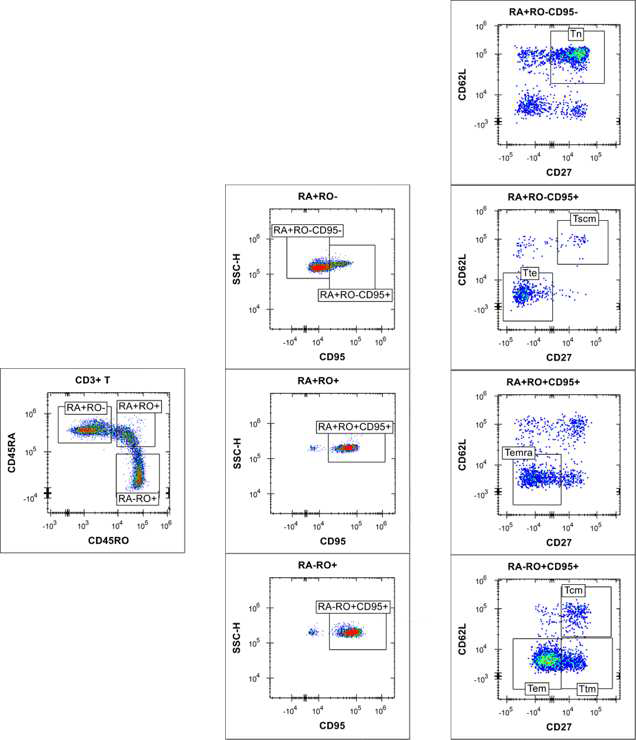
Figure 5. Gating of T memory cells at plate level. Image Credit: Sartorius
Without the need for single stain compensation, the template contains validated compensation metrics that apply instantaneously to the data analysis. All populations were differentiated at the plate level, not at the well level, for the gating strategy.
The scatter plot was used to separate the cells and beads due to differences in size and granularity. Based on bead intrinsic fluorescence in the RL1 and RL2 channels of the iQue®, the two different cytokine capture beads (IFN γ and IL-10) were separated.
RL1 is also involved in cell detection via CD95, but it has no effect on bead classification. 2D plots with varied CD markers/viability staining signals were used to separate cells. In CD3+ T cell populations, distinct T naïve/memory/effector cell subsets have been identified (Figures 4 and 5). Boolean logic gates were used to recognize each corresponding CD4+ and CD8+ T naïve | memory | effector cell subset (data not shown).
Standard curves to quantify the amount of secreted IFNγ and IL-10 were created using a 4-parameter curve fit with 1/Y2 weighting, and the linear range (represented by bold lines in Figure 6) for each standard curve was evaluated using Forecyt®.
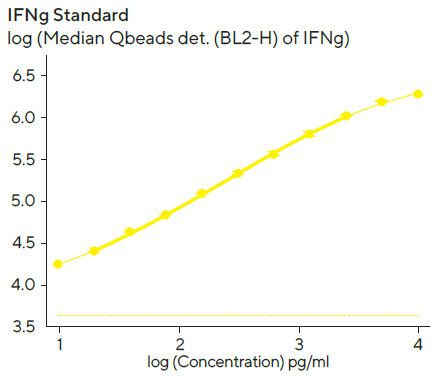
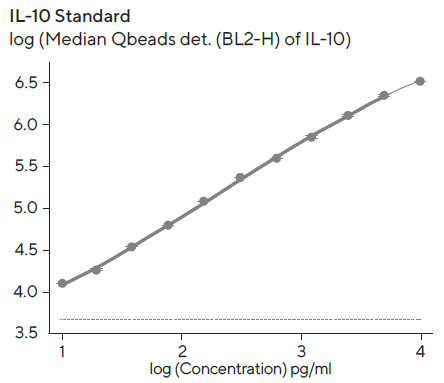
Figure 6. IFN and IL-10 standard curves. Image Credit: Sartorius
Standard curves were created with the kits provided standard and 1:2 serial titration with a top concentration of 10,000 pg/mL per standard protein. The linear ranges for standards were as follows: IL-10, 10–6, 400 pg/mL; IFN, 10–2, 100 pg/mL. The detection ranges were larger than the linear range, and the dashed line denotes the fluorescent background at zero standard concentration.
Results and discussion
Forecyt® was used to evaluate the T cell expansion time course data and create series graphs from the POC studies for each donor. TCM or TSCM frequency was increased in culture media supplemented with cytokine cocktails containing IFNß or IL-21 cytokine, respectively.
With CD3/CD28 Dynabeads® treatment, the data was presented as a fold increase over the control wells. Donor 1’s results on Day 5 are shown in Figure 7. TCM (particularly cytotoxic CD8+ TCM) frequency increased in six IFNß cocktails, while TSCM (notably helper CD4+ TSCM) frequency increased in four IL-21 cocktails (Figure 7A).
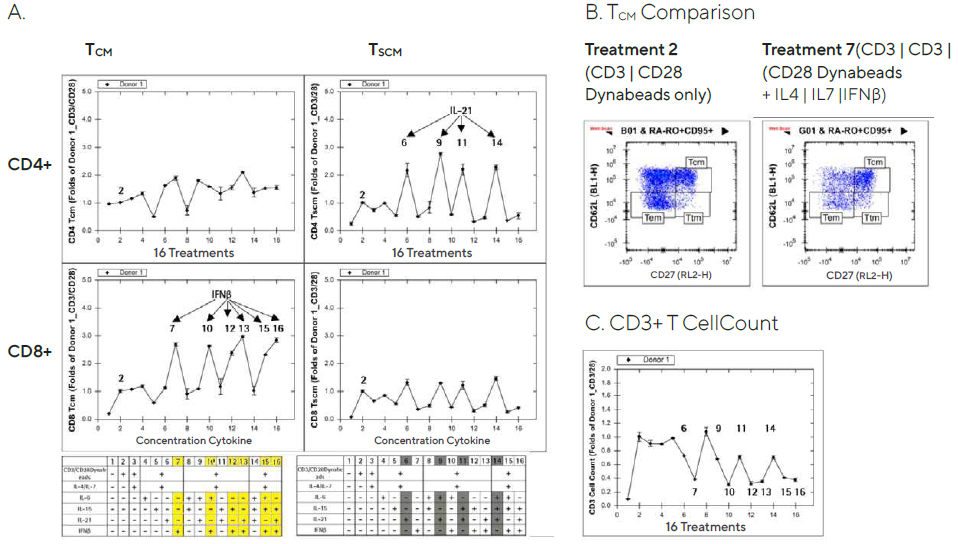
Figure 7. Increased T memory cell frequency after ex vivo expansion in cytokine supplemented media. Image Credit: Sartorius
Though it has been reported that type interferons, including IFNß, act directly on CD8+ T cells to allow clonal expansion and memory formation, the full mechanism of IFNß’s selective effect on CD8+ TCM and IL-21’s selective effect on CD4+ TSCM is unknown.8
TCM cell frequency was elevated in the wells treated with a cocktail containing IFNß compared to the wells treated with CD3/CD28 Dynabeads® alone, as shown in Figure 7B’s 2D plots (CD27 vs. CD62L).
In addition, CD3+ T cell counts in culture supplemented with cytokine cocktails containing IFNß decelerated cell proliferation by about 50%. (Figure 7C). TCM and TSCM cell frequencies were only mildly affected by the treatments on Day 2 post-treatment, in the same direction as on Day 5, and Day 8 produced similar results (data not shown).
The Forecyt® software was used to determine the temporal analysis of IL-10 and IFN secretion. Donor 1’s results are shown in Figure 8. The results were expressed as a fold increase over the control wells with only CD3/CD28 Dynabeads® treatment.
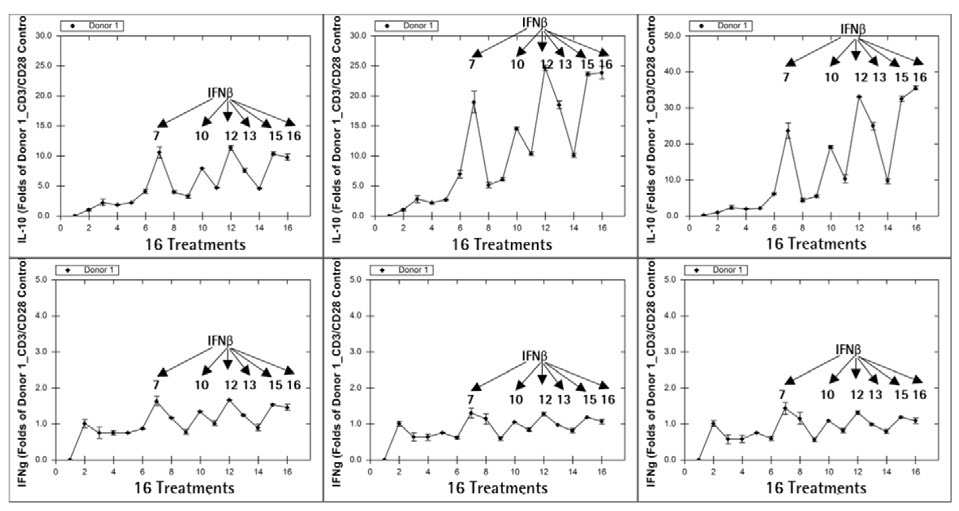
Figure 8. IL-10 secretion correlates with CD8+ TCM cell frequency. Image Credit: Sartorius
From Day 2 to Day 8, six cytokine cocktails comprising IFNß in the culture enhanced both IL-10 and IFN secretion. The IL-10 secretion pattern was strikingly similar to the CD8 TCM frequency pattern (Figure 7A).
Even on Day 2, when most cocktail treatments had no significant increase in TCM (data not shown), IL-10 secretion followed the same pattern as on Day 5. This suggests that an increase in immune cell IL-10 secretion could signal an increase in TCM frequency in the coming days.
Additional findings from the POC screening studies indicate that CD8+ T memory cell frequencies vary from donor to donor, which is unsurprising given that not all patients are expected to be candidates for adoptive T cell therapy. As an example, the results from Day 5 are shown (Figure 9).
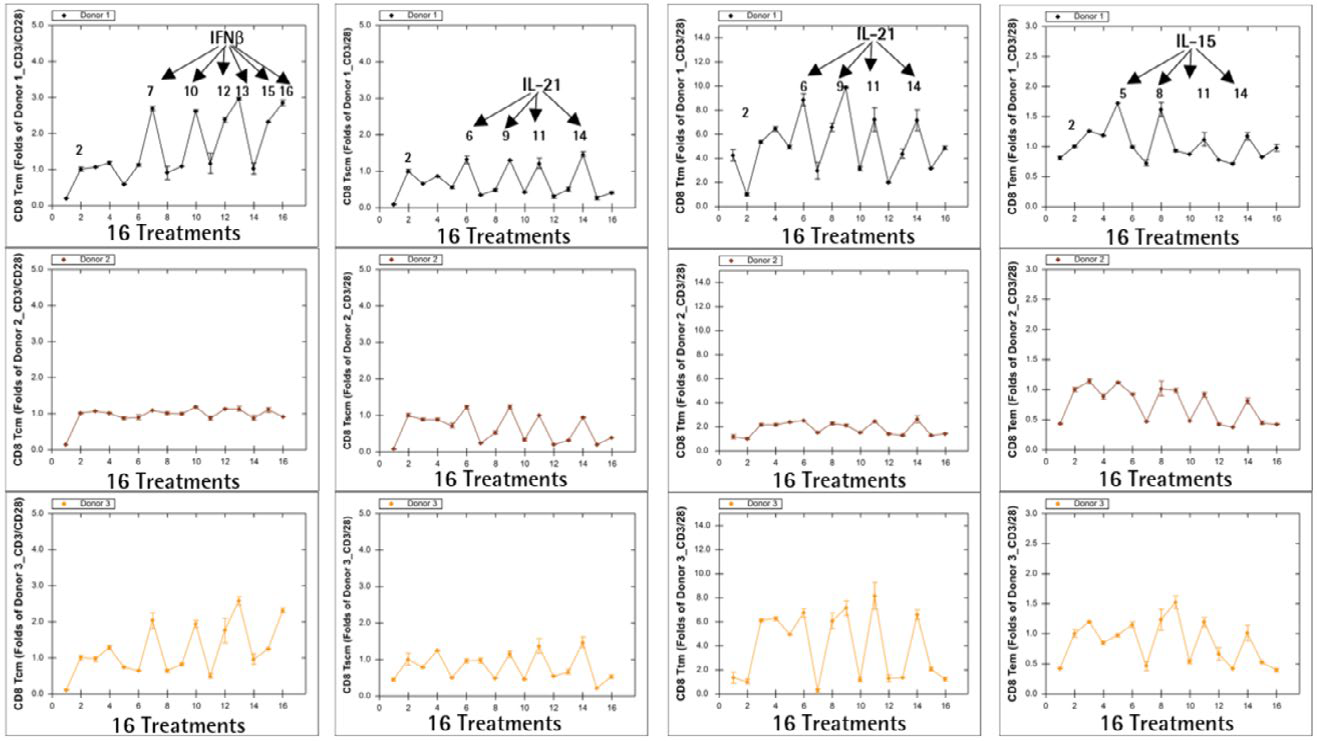
Figure 9. Donor-to-donor variation in ex vivo T cell expansion. Image Credit: Sartorius
Donors 1 and 3 had a greater frequency of CD8+ TCM when cultured in IFNß-containing media, higher frequencies of CD8+ TSCM and CD8+ Ttm when cultured in IL-21-containing media, and a higher frequency of CD8+ TEM when cultured in IL-15-containing media.
All media combinations, on the other hand, had only a minor or no effect on the frequency of T cell memory subsets in Donor 2, implying that donor sensitivities to various cytokine treatments in ex vivo T cell expansion are donor-dependent.
Conclusion
This application note shows how to use the iQue® Human T Cell Memory Kit’s simple assay and analysis workflow with a screening model that, regardless of the complexity of T cell biology, can be beneficial for adoptive T cell therapy workflows. By combining cells and beads and multiplexing measurements in each assay well, the kit is designed to be simple to use. It has the following distinct advantages:
- Multiplexed cell and secreted cytokine measurement in a single assay, a significant improvement over traditional immunology workflows, which typically require multiple assays to be run on different platforms.
- With integrated Forecyt® software, the iQue® advanced flow cytometry platform provides a single platform and data analysis package. Simplifies data acquisition and analysis workflows, including multi-plate analysis, and eliminates data synchronization problems. It also transforms data into actionable results in a timely manner.
- Color compensation is not required in this simplified Plug-and-Play assay workflow. For ease of use, the majority of the reagents are pre-mixed. Validated compensation metrics are included in the assay template and are applied to data analysis automatically. By referring to the corresponding standard curve, cytokine concentration is also automatically interpolated.
- In a single high-content miniaturized assay, spatial-temporal analysis of T memory cell phenotypes and functions at various stages. This format conserves valuable samples, lowers reagent costs and improves data integrity.
- Additional cytokine measurements can be selected from the validated iQue® Human T Cell Companion Kits: IL-2, IL-6, IL-13, IL-17A, GM-CSF, and TNF.
References
- Farber DL, Yudanin NA, and Restifo NP, Human Memory T cells: generation, compartmentalization and homeostasis. Nature Reviews Immunology, Jan;14(1) (2014)
- Mahnke YD, et al. The who’s who of T-cell differentiation: Human Memory T-cell subsets. European Journal of Immunology, (43) (2013)
- Petersen CT, et al. Improving T-cell expansion and function for adoptive T-cell therapy using ex vivo treatment with PI3k inhibitors and VIP Antagonists. Blood Advances, Feb;(2)3 (2018)
- Teschner D, et al. In Vitro Stimulation and Expansion of Human Tumour-Reactive CD8+ Cytotoxic T Lymphocytes by Anti-CD3 | CD28 | CD137 Magnetic Beads. Scandinavian Journal of Immunology, (74) (2011)
- Medvec AR, et al. Improved Expansion and In Vivo Function of Patient T Cells by a Serum-free Medium. Molecular Therapy: Methods & Clinical Development, Mar;(8) (2018)
- Cieri N, et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood, Jan;121(4);24 (2013)
- Golubovskaya V, and Wu L, Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy. Cancers, 8(36) (2016)
- Kolumam GA, et al. Type I Interferons Acts Directly on CD8 T Cells to Allow Clonal Expansion and Memory Formation in Response to Viral Infection. Journal of Experimental Medicine, 202(5) (2005)
About Sartorius

Sartorius is a leading international pharmaceutical and laboratory equipment supplier. With our innovative products and services, we are helping our customers across the entire globe to implement their complex and quality-critical biomanufacturing and laboratory processes reliably and economically.
The Group companies are united under the roof of Sartorius AG, which is listed on the Frankfurt Stock Exchange and holds the majority stake in Sartorius Stedim Biotech S.A. Quoted on the Paris Stock Exchange, this subgroup is comprised mainly of the Bioprocess Solutions Division.
Innovative technologies enable medical progress
A growing number of medications are biopharmaceuticals. These are produced using living cells in complex, lengthy and expensive procedures. The Bioprocess Solutions Division provides the essential products and technologies to accomplish this.
In fact, Sartorius has been pioneering and setting the standards for single-use products that are currently used throughout all biopharmaceutical manufacturing processes.
Making lab life easier
Lab work is complex and demanding: Despite repetitive analytical routines, lab staff must perform each step in a highly concentrated and careful way for accurate results.
The Lab Products and Services Division helps lab personnel excel because its products, such as laboratory balances, pipettes and lab consumables, minimize human error, simplify workflows and reduce physical workloads
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.