Sponsored Content by SartoriusReviewed by Alex SmithFeb 10 2023
Fragment-based drug discovery (FBDD) has gained widespread recognition as a valuable approach in the search for lead drug candidates in drug discovery programs. It is utilized in conjunction with structure-based drug design and high-throughput screening.
In 2021, the FDA approved a majority of non-protein-based drugs, over 70%. This highlights the importance of evaluating kinetics and affinity for various molecular sizes early in the selection process.
Biophysical technologies facilitate the detection and characterization of binding events, including low-affinity interactions of low molecular weight compounds.
SPR is a critical technology for binding event detection in pharma and biotech. It offers high sensitivity and throughput, enabling complete fragment screening of thousands of compounds in a matter of weeks per target.
Traditional SPR screening for low-affinity compounds using fixed concentration injections can pose difficulties, such as weak affinities (ranging from µM to mM) and low molecular weights.
These challenges often lead to decisions based on inadequate sensorgrams, which can be small and square-shaped.
Given these limitations, there is a requirement for methods that can overcome these restrictions, improve hit confidence, reduce timelines, and provide screening groups with more extensive datasets for more thorough analysis.
The OneStep® Injections unique to Octet® SF3 improve SPR screening with continuous analyte titration, providing rich datasets for confident and speedy hit characterization.
OneStep® injections allow users to obtain kinetic rate constants, affinity, and ligand efficiency directly from the initial screen, streamlining the traditional SPR workflow into one assay.
Using a well-characterized small molecule system, standard multi-cycle kinetics are inadequate from a single-concentration injection. Reliable kinetics and affinity can be obtained with less data.
Moreover, OneStep® injections can reduce the number of measurements necessary for accurate kinetics and affinity determination to a single measurement, increasing sample throughput and conserving sample material.
This eliminates the obstacle for SPR as a tool in large screens and speeds up the advancement towards further assay development of potential therapeutics.
Methods
Instrument and reagents
All assays were carried out using an Octet® SF3 SPR system with phosphate-buffered saline with 0.05% Tween 20 (PBS-T) at pH 7.4 as a running buffer. The temperature for all assays was 25 °C unless specified otherwise.
PBS was obtained from Gibco. EDC was obtained from Thermo Fisher Scientific. The remaining reagents were purchased from Sigma Aldrich and prepared in-house.
Kinetics and affinity determination
Carbonic anhydrase II
Using the standard amine coupling technique, bovine carbonic anhydrase II (CAII) was attached to an Octet® SPR PCH Sensor Chip.
Briefly, the flow cells 1, 2, and 3 were treated with a solution of 50% 0.4 M EDC and 50% 0.1 M NHS at a flow rate of 10 µL/min for seven minutes.
CAII was immobilized on an Octet® SPR PCH Sensor Chip using EDC/NHS mixture and CAII in sodium acetate pH 5.0. The surface was activated using EDC/NHS mixture and CAII was then attached. Deactivation was done using 1 M ethanolamine HCl pH 8.5.
CAII was immobilized on Octet® SPR PCH Sensor Chip using amine coupling. This allowed for the determination of the kinetics and affinity of CAII.
Bovine CAII was immobilized on Octet® PCH Sensor Chip using standard amine coupling. EDC and NHS mixture was injected for seven minutes at 10 µL/min in flow cells 1, 2, and 3.
CAII immobilized on Octet® PCH Sensor Chip using standard amine coupling method. EDC and NHS mixture injected, followed by CAII, and deactivated with ethanolamine HCl.
Furosemide and acetazolamide were tested using an Octet® SPR system. Furosemide (330 Da) was diluted 3-fold (10 µM---0.123 µM) and 10-fold (100 µM---0.01 µM) and acetazolamide was at a single concentration. All were dissolved in PBS-T buffer.
Samples were analyzed using a mixed format sample rack on an Octet® SPR system. A 3% sucrose solution in PBS-T was used as a reference for OneStep® injections. The sample rack was sealed using a resealable septum and placed in the sample tray set to 20 °C.
The Octet® SF3 system was primed using PBS-T running buffer and the sensor chip was hydrated and conditioned using PBS-T.
Fixed concentration injections were performed using a standard assay format with an association time of 60 seconds and dissociation time of 140 seconds at 100 µL/min.
Fixed concentration injections were used with OneStep® with 60-second association and 140-second dissociation at 100 µL/min.
The buffer blank injection ensured data accuracy by subtracting any non-specific binding. The 1:1 interaction model was used to fit all multi-cycle kinetics data for determining kinetics and affinity parameters.
Data for fixed concentration injections and OneStep® injections was analyzed using a 1:1 interaction model for kinetics and affinity determination.
Results and discussion
The goal was to show that typically fixed concentration injections (FCI) employed in SPR screens, which deliver a flow of uniform analyte concentration over the sensor chip surface, are frequently beyond the limits that induce sufficient curvature for kinetic analysis as a single concentration.
In contrast to a single-concentration injection, OneStep® injections offer acceptable kinetic curvature across a significantly larger range. They can produce kinetic and affinity constants that compare well to those generated from typical multi-cycle kinetic experiments.
Moreover, because the affinity of the drug-therapeutic is sometimes unknown, the flexibility in setting the initial analyte concentration choice is considered.
Determining reliable kinetic and affinity parameters from a minimal data set
Previous attempts to find the smallest data set necessary for multi-cycle kinetics have revealed that substantial curvature is required to determine correct kinetics in a confidently measurable area.3 Even so, preparatory screening is essential to identify the appropriate concentrations.
The well-characterized specific molecular interaction between furosemide and carbonic anhydrase II has historically had literature values in the 500-1000 nM range.2, 4, 5
Table 1 shows an initial study of the kinetics and affinity determined by fixed concentration injections ranging from 0.01-100 µM, and Figure 1A—C shows the accompanying sensorgram.
The greatest fixed concentration injection of 100 M displays an excessively quick association phase, as illustrated in Figure 1A and emphasized in Figure 1B. Just a few data points occur during the kinetic phase.
The binding responses at the lowest fixed concentration injections of 0.01 M and 0.1 µM can be interpreted as linear and lack precise information about the reaction kinetics.
The calculated affinity values of 5.8 nM, 105 nM, and 2050 nM for 0.01, 0.1, and 100 µM fixed concentration injections, respectively, reflect this absence of meaningful kinetic information.
Figure 1C depicts fixed concentration injections of 1 and 10 µM, which exhibit significant curvature and approach equilibrium, allowing precise kinetic rate constants to be obtained.3 Affinity values of 552 nM and 851 nM for 1 µM and 10 µM are broadly in line with published values.
Table 1. Source: Sartorius
| |
Concentration (μM) |
ka (M–1s–1) |
kd (s–1) |
KD (nM) |
| Furosemide |
100 |
2.99 * 104 |
0.0614 |
2050 |
| |
10 |
6.22 * 104 |
0.0529 |
851 |
| |
1 |
9.21 * 104 |
0.0509 |
552 |
| |
0.1 |
4.93 * 105 |
0.0518 |
105 |
| |
0.01 |
8.60 * 106 |
0.0498 |
5.8 |
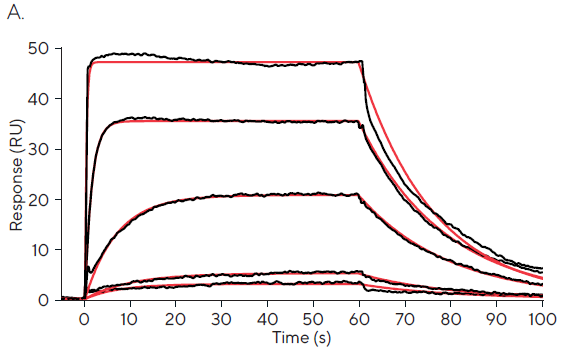
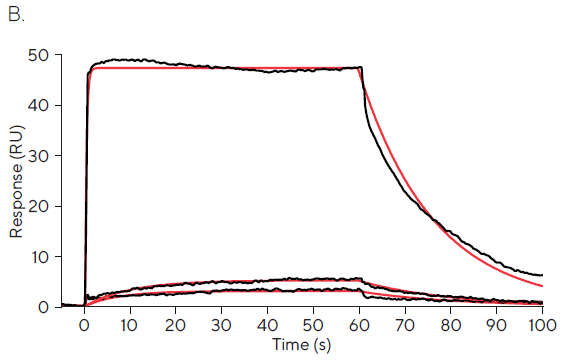
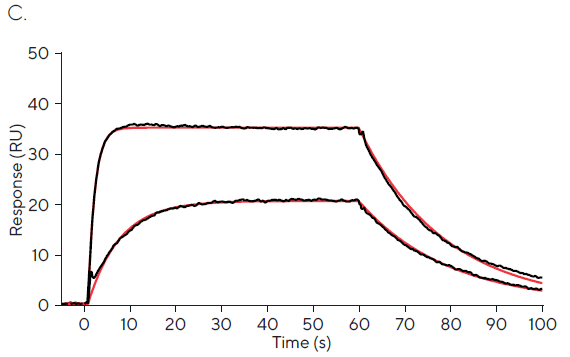
Figure 1. Image Credit: Sartorius
Using OneStep® injections, a single injection of each Furosemide concentration was then delivered across the same surface.
It should be noted that repeated OneStep® injections are not necessary for routine screening, and the reason for doing several injections here was to discover the tolerance levels between fixed concentration injections and OneStep® injections.
Table 2 shows the kinetic parameters for local fits of each concentration, and Figure 2 shows the associated sensorgrams.
Table 2. Source: Sartorius
| |
Concentration (μM) |
ka (M–1s–1) |
kd (s–1) |
KD (nM) |
| Furosemide |
100 |
5.36 * 104 |
0.0448 |
835 |
| |
10 |
8.49 * 104 |
0.0529 |
623 |
| |
1 |
8.14 * 104 |
0.0539 |
660 |
| |
0.1 |
1.20 * 105 |
0.0518 |
430 |
| |
0.01 |
1.00 * 106 |
0.0100 |
10 |
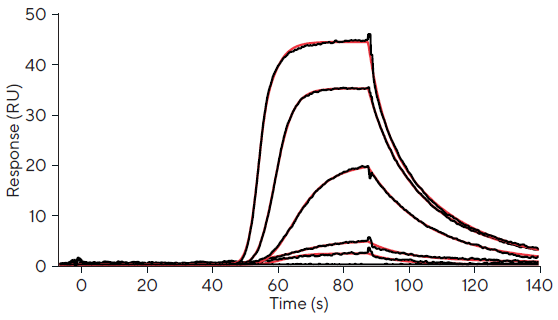
Figure 2. Image Credit: Sartorius
Unlike fixed concentration injections, OneStep® injections exhibit curvature across the concentration series, enabling for the determination of valid kinetic rate constants over a far larger range of concentrations.
This is shown in a better estimate of kinetic and affinity parameters for values outside of the fixed concentration injection range of 0.1 µM and 100 µM.
Unlike the fixed concentration injection for 100 µM, the OneStep® injection has substantial curvature and enables the determination of a valid kinetics and affinity value consistent with published values (835 nM).
This improvement may also be seen in the 0.1 µM OneStep® injection, which has an affinity value of 430 nM compared to the fixed concentration injection's 105 nM. In comparison to typically fixed concentration injection, a single OneStep® injection is sufficient to ascertain precise association kinetics.

 Download the full application note
Download the full application note
References
- Karlsson R and Larsson A. Methods Mol Biol. 2004; 248: 389-415
- Myszka D. Anal Biochem. 2004; 329 (2): 316–323
- Onell A and Andersson K. J. Mol Recognit. 2005;18(4): 307-317
- Papalia G. Anal Biochem. 2006; 359(1): 94–105
- Rich R. Anal Biochem. 2010; 407(2): 270–277
About Sartorius

Sartorius is a leading international pharmaceutical and laboratory equipment supplier. With our innovative products and services, we are helping our customers across the entire globe to implement their complex and quality-critical biomanufacturing and laboratory processes reliably and economically.
The Group companies are united under the roof of Sartorius AG, which is listed on the Frankfurt Stock Exchange and holds the majority stake in Sartorius Stedim Biotech S.A. Quoted on the Paris Stock Exchange, this subgroup is comprised mainly of the Bioprocess Solutions Division.
Innovative technologies enable medical progress
A growing number of medications are biopharmaceuticals. These are produced using living cells in complex, lengthy and expensive procedures. The Bioprocess Solutions Division provides the essential products and technologies to accomplish this.
In fact, Sartorius has been pioneering and setting the standards for single-use products that are currently used throughout all biopharmaceutical manufacturing processes.
Making lab life easier
Lab work is complex and demanding: Despite repetitive analytical routines, lab staff must perform each step in a highly concentrated and careful way for accurate results.
The Lab Products and Services Division helps lab personnel excel because its products, such as laboratory balances, pipettes and lab consumables, minimize human error, simplify workflows and reduce physical workloads.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.