Sponsored Content by SartoriusReviewed by Alex SmithMar 7 2022
When T-cells are activated, naive CD4+ cells will differentiate into various types of T helper (Th) cells. One notable factor influencing this differentiation is the cytokine milieu present during antigen engagement. This differentiation is also driven by autocrine mechanisms.
Th subsets are linked to adaptive immune responses in physiological processes. Specific pathogens and Th cell dysregulation are also key contributors to allergic and autoimmune diseases.
It is possible to identify Th subsets – at least in part – by analyzing their unique cytokine secretion profile.
The iQue Qpanels® T Helper Kits from Sartorious have been designed to facilitate the measurement of Th cells’ cytokine secretion. These pre-configured multiplexed bead-based kits can be used to quantitate Th1 and Th2 (4-, 6-, or 9-plex) or Th1, Th2, and Th17 (7-plex) associated cytokines in cell supernatants.
As they are miniaturized assays, the iQue Qpanels® T Helper Kits only require 10 μL for each sample. Reagents are pre-mixed to support ease of use and streamlined workflows.
Data acquisition is undertaken on the iQue® Advanced Flow Cytometry Platform using 96-, 384-, or 1536-well plates.
The full range of pre-mixed reagents and cytokine standards help streamline workflows, while templated data analysis allows all parameters to be automatically generated. These include event gates, the gating strategy, four or five parameter logistic standard curves and sample quantitation.
These factors combine to facilitate the rapid enumeration of Th cell cytokine profiles.
The multiplexed assay is able to simultaneously detect all proteins via a straightforward, single-wash protocol that offers users genuinely actionable results in a short time – just over 3 hours from sample addition to the generation of quantitated cytokine profiles.
Assay principles
iQue Qpanels® T Helper Kits feature pre-mixed multiplexed cytokine capture beads that have been coated with antibodies directed against Th cytokines. Each of these beads has been designed to bind one specific Th analyte and has been encoded with varying intensities of two different fluorescent colors.
This encoding enables the different beads to be tracked during quantitation on the iQue® platform, allowing users to view which cytokines have been captured by each specific bead.
It is also important to note that each kit includes pre-mixed lyophilized standards to enable a rapid standard curve setup.
The iQue Forecyt® software is able to calculate four or five parameter logistic curves, enabling quantitation of a sample’s Th cytokine secretion levels. This is done in a multiplexed system to allow for simultaneous binding and detection at picograms per milliliter (pg/mL) levels.
Assay workflow
All of the kit’s components are pre-mixed for maximum ease of use. Users are simply required to dissolve the cytokine standards and dilute the capture beads prior to commencing the assay.
This is done by dissolving the pre-mixed lyophilized standards, preparing a standard curve, and then diluting the pre-mixed capture beads with the included assay buffer.
Next, 10 μL of the samples or standards should be combined with 10 μL of the pre-mixed capture beads. This mixture should be incubated for a total of 1 hour before 10 μL of the pre-mixed detection reagent is added and the resulting mixture incubated for another 2 hours.
One wash should be performed at the end of incubation before resuspending the beads and reading the result on the iQue® Advanced Flow Cytometry Platform.
The whole protocol takes approximately 3.5 hours. Plate reads take less than 20 minutes for 384-well or 10 minutes for 96-well plates, irrespective of the iQue Qpanel® (4-, 6-, 7-, or 9-plex) kit employed.
Methods
Cell culture
These particular studies saw peripheral blood mononuclear cells (PBMCs) from three donors being cultured in media, which included human sera and IL-2.
Cells were then stimulated using anti-CD3 | CD28 beads, while control cells did not include any stimulation beads. Supernatants were acquired at 1-, 2-, and 3-days post-stimulation. Prior to being assayed, these were kept frozen at -80 °C.
Cytokine quantitation
The 7-plex Th1 | Th2 | Th17 iQue Qpanel® kit (IL-2, IL-4, IL-6, IL-10, IL-17A, IFNγ and TNFα) was used with the cell culture supernatants. This process included a number of steps.
First, the lyophilized standard was dissolved and serially diluted 1:2 to prepare a 12-point dilution series. A total of 10 μL of each individual dilution of the standard curve was transported to a fresh 96-well assay plate.
Supernatants were then thawed before 10 μL of each was added to the assay plate. This was done in triplicate for each sample.
The pre-mixed capture beads were diluted to their working volumes before 10 μL was added to each well, whether this well included supernatant or standard. The contents of the wells were mixed before being incubated for 1 hour at room temperature.
A total of 10 μL of pre-mixed detection reagent was added into each individual well before this was mixed and incubated for a further 2 hours at room temperature.
Next, 50 μL of buffer – designed to function as a wash – was added to each well. The plate was centrifuged for a total of 5 minutes at 1100 xg before being aspirated using a BioTek ELx405 plate washer. After the aspiration had been completed, 10 μL buffer was added to resuspend the beads.
Finally, the 96-well plate was read using an iQue® Screener PLUS (VBR configuration). This reading took less than 10 minutes.
Using the iQue Forecyt® software, it was possible to ascertain curve fits for the cytokine standards.
These fits were then used to quantitate the number of cytokines secreted by the PBMCs by converting the median fluorescence intensity (MFI) associated with each bead’s captured analyte to determine the actual secreted concentrations.
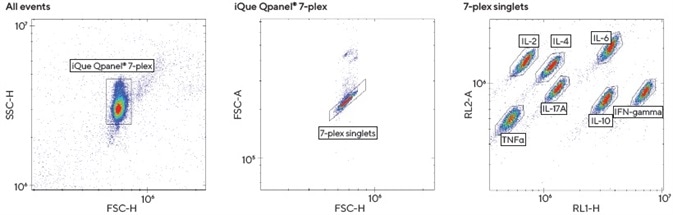
Image Credit: Sartorius
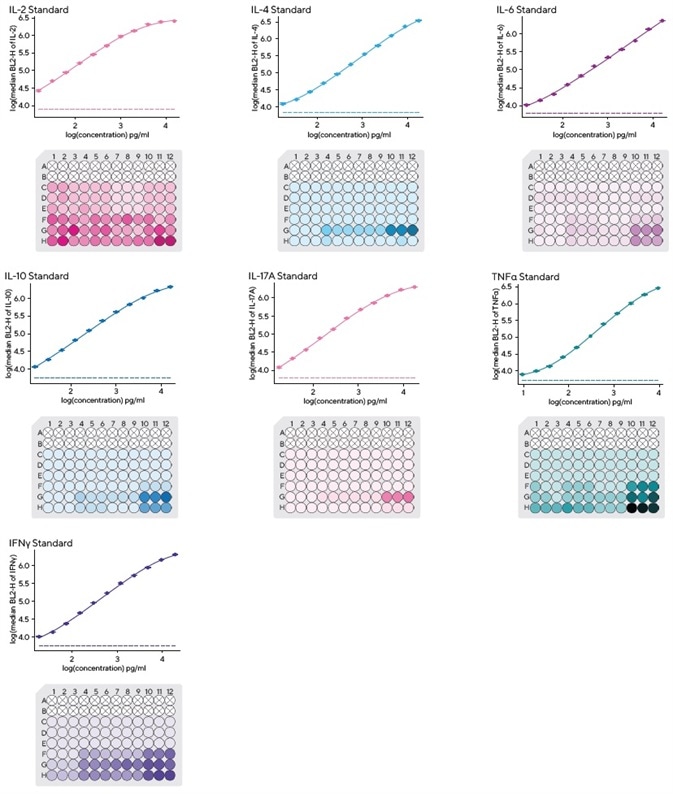
Image Credit: Sartorius
Results
It was observed that there was a degree of donor to donor variation and some temporal differences in secreted cytokine levels. The addition of exogenous IL-2 to the media helped encourage T-cell growth and viability.
It was noted that this was detectable in control wells, but increased IL-2 production (approximately 2–3 fold over control) was observed in stimulated cultures from all three donors.
The complete set of donor PBMCs exhibited a Th1-type response, secreting IFNγ and TNFα post-stimulation. Secretion was found to increase in a time-dependent manner on days 2 and 3.
It was also observed that one of the donors (donor 2) demonstrated more robust Th2 (IL-4, IL-6, and IL-10) and Th17 (IL-17A) responses than the others.
Discussion
iQue Qpanels® represent a straightforward, accessible means of profiling Th cell subsets. These kits can be used to efficiently screen secreted proteins from T cells in order to quantitate multiple cytokines.
Their simple, pre-mixed format requires minimum hands-on time while ensuring that maximum data is obtained.
This data serves to highlight the application of iQue Qpanels® in T cell screening and the panels’ role in the analysis of effector function and identification of Th subsets. The data also demonstrates donor and time-dependent cytokine secretion.
Streamlined, pre-mixed Th panels include:
- 4-plex Th1 | Th2 panel: IL-4, IL-6, IFNγ, TNFα
- 6-plex Th2 | Th2 panel: IL-2, IL-4, IL-6, IL-10, IFNγ, TNFα
- 7-plex Th1 | Th2 | Th17 panel: IL-2, IL-4, IL-6, IL-10, IL-17A, IFNγ, TNFα
- 9-plex TH1 | Th2 panel: IL-2, IL-4, IL-6, IL-10, IL-12(p70) IL-13, IFNγ, TNFα, GM-CSF
The kits’ standards, capture beads, and detection reagent are all pre-mixed, enabling a quick and simple assay setup. The iQue Forecyt® acquisition and analysis templates also ensure rapid data analysis and quantitation.
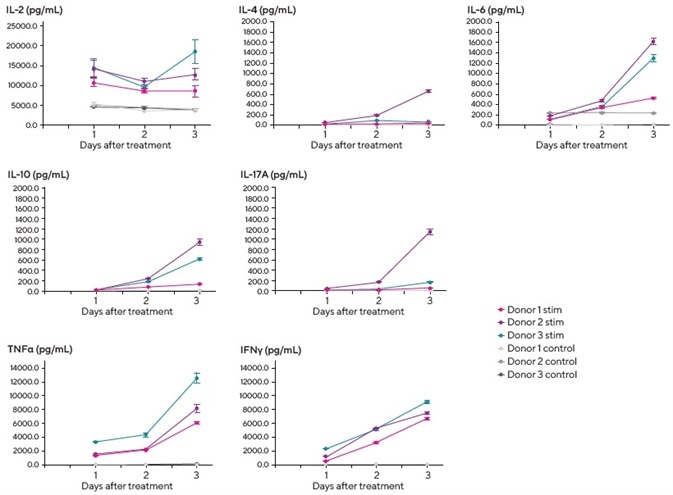
Image Credit: Sartorius
Acknowledgments
Produced from materials originally authored by Mark Carter and John O’Rourke from Essen BioScience, Inc., part of the Sartorius Group.
About Sartorius

Sartorius is a leading international pharmaceutical and laboratory equipment supplier. With our innovative products and services, we are helping our customers across the entire globe to implement their complex and quality-critical biomanufacturing and laboratory processes reliably and economically.
The Group companies are united under the roof of Sartorius AG, which is listed on the Frankfurt Stock Exchange and holds the majority stake in Sartorius Stedim Biotech S.A. Quoted on the Paris Stock Exchange, this subgroup is comprised mainly of the Bioprocess Solutions Division.
Innovative Technologies Enable Medical Progress
A growing number of medications are biopharmaceuticals. These are produced using living cells in complex, lengthy and expensive procedures. The Bioprocess Solutions Division provides the essential products and technologies to accomplish this.
In fact, Sartorius has been pioneering and setting the standards for single-use products that are currently used throughout all biopharmaceutical manufacturing processes.
Making Lab Life Easier
Lab work is complex and demanding: Despite repetitive analytical routines, lab staff must perform each step in a highly concentrated and careful way for accurate results.
The Lab Products and Services Division helps lab personnel excel because its products, such as laboratory balances, pipettes and lab consumables, minimize human error, simplify workflows and reduce physical workloads
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.