Claudin is a protein family that contains at least 27 members and it was first identified by Furuse in 1998. It is a four-pass transmembrane protein that has been expressed at the epithelial-endothelial tight junction and is a component of tight junction strands, which are important for signal transduction and cell polarity.
Claudin18.2, a known subtype of Claudin18, is weakly expressed in differentiated epithelial cells of the gastric mucosa, but highly expressed in a variety of malignant tumor tissues, such as pancreatic cancer, gastric cancer, ovarian cancer, lung cancer, cholangiocarcinoma, among others.
As a result, it has become a popular target for immunotherapy, such as monoclonal antibodies, bispecific antibodies, ADCs and CAR-T therapy.
Limited by the surface area of the cell membrane and the potential cytotoxicity of overexpressed membrane proteins, the expression level of recombinant four-pass Claudin18.2 proteins is much lower compared to that of soluble proteins.
Moreover, it is essential to screen appropriate detergents or lipids to maintain spatial conformation and protein activity in the extraction and purification process. Hence, that makes it very difficult to achieve Claudin18.2 antigen with high purity, native conformation and activity. This tends to seriously hinder the development of drugs and therapies targeting Claudin18.2
ACROBiosystems has successfully generated full-length Claudin18.2-VLP and Claudin18.2-DDM/CHS with native structure using multi-pass transmembrane protein technology platforms, and their high biological activity is verified by ELISA/BLI/SPR. This satisfies the criteria of drug development.
At the same time, a high-quality integrated SPR&BLI service using Claudin18.2 is available for antibody screening, characterization and consistency assessment to support drug development and clinical declaration.
Structure of Claudin18.2 (AlphaFold structure prediction). Video Credit: ACROBiosystems
Product list
Table 1. Source: ACROBiosystems
| Molecule |
Cat. No. |
Production Description |
Application |
| Claudin18.2 |
CL2-H5547 |
Human Claudin-18.2 Full Length Protein-VLP (HEK293) |
Immunization
ELISA
SPR
BLI
Cell-based assay
CAR detection |
| Claudin18.2 |
CL2-H52P7 |
Human Claudin-18.2 Full Length Protein-VLP (HEK293, stable cell pool) |
| Claudin18.2 |
CL2-HF218 |
Fluorescent Human Claudin-18.2 Full Length Protein-VLP (HEK293) |
| Claudin18.2 |
CL2-H5546 |
Human Claudin-18.2 Protein, His Tag (active membrane protein) |
Immunization
ELISA
SPR
BLI |
| Claudin18.2 |
CL2-H82E3 |
Biotinylated Human Claudin-18.2 Protein, His,Avitag™ (active membrane protein) |
| Claudin18.2 |
CL2-H5587 |
Human Claudin-18.2 Protein, His,Twin-Strep Tag (active membrane protein) |
| VLP |
VLP-H52C5 |
Virus-Like Particle (VLP) isotype control |
Virus-Like Particle (VLP) isotype control |
| Buffer |
DC-11 |
200x DDM CHS buffer |
Maintain solubilization and activity of membrane proteins Dilute membrane proteins or antibodies Prepare running buffer in SPR or BLI assay, etc. |
Product feature
The ELISA/SPR/BLI procedures listed below are accessible for free.
Full-length Claudin18.2-VLP (CL2-H5547)
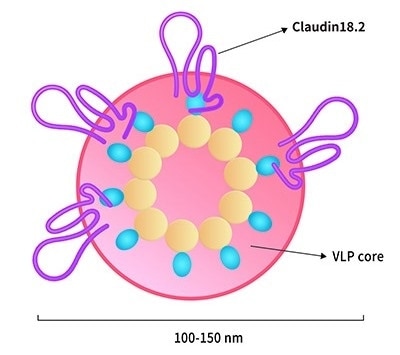
Image Credit: ACROBiosystems
Advantages
- Cellular and humoral immune responses are improved
- Full-length Claudin18.2
- Suitable for CAR detection/immunization/ELISA/SPR/BLI/cell-based assay
- Due to its size of 100–200 nm, it provides the best target presentation to APC and phage display
Exclusive Claudin18.2-DDM/CHS (CL2-H5546, CL2-H82E3)
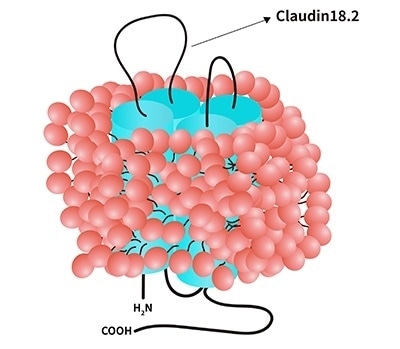
Image Credit: ACROBiosystems
Advantages
- Suitable for immunization/ELISA/ BLI/SPR/
- Can be precisely measured
- Biotinylated Claudin18.2 (CL2-H82E3) is now available
- Claudin18.2 with entire conformation
- In the presence of DDM/CHS, it has a high solubility (DC-11)
Claudin18.2-VLP
Correct assembly validated by SEM
Under an electron microscope, full-length Claudin18.2-VLP (Cat. No.CL2-H5547) was examined to confirm that it was appropriately built.
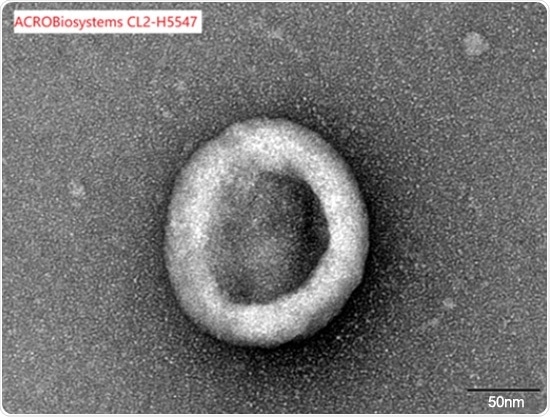
Image Credit: ACROBiosystems
Good bioactivity validated by ELISA
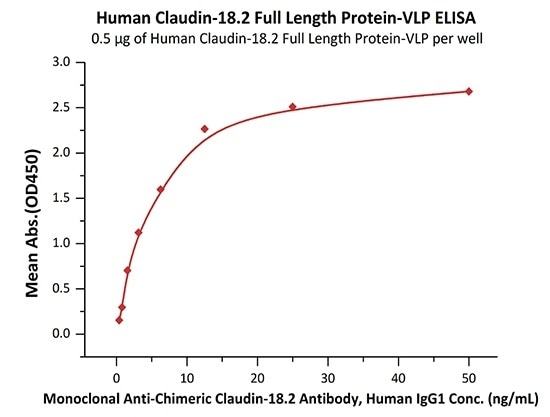
Immobilized Human Claudin-18.2 Full Length Protein-VLP (Cat. No. CL2-H5547) at 5 μg/mL (100 μL/well) can bind Monoclonal Anti-Chimeric Claudin-18.2 Antibody, Human IgG1 with a linear range of 0.8–3 ng/mL (QC tested). Image Credit: ACROBiosystems
High affinity validated by SPR
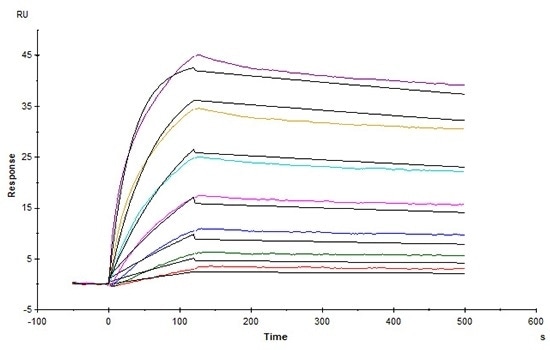
Human Claudin-18.2 Full Length Protein-VLP (Cat. No. CL2-H5547) captured on CM5 Chip via Anti-Claudin-18.2 antibody can bind Anti-Claudin-18.2 antibody with an affinity constant of 1.24 nM as determined in an SPR assay (Biacore T200) (Routinely tested). Image Credit: ACROBiosystems
Suitable for CAR detection
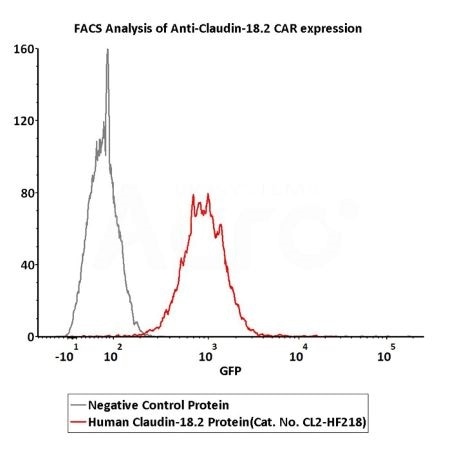
2e5 of Anti-Claudin-18.2 CAR-293 cells were stained with 100 μL of 3 μg/mL of Fluorescent Human Claudin-18.2 Full Length Protein-VLP (Cat. No.CL2-HF218) and negative control protein respectively, FITC signals was used to evaluate the binding activity (Routinely tested). Image Credit: ACROBiosystems
Exclusive Claudin18.2-DDM/CHS
High bioactivity validated by ELISA
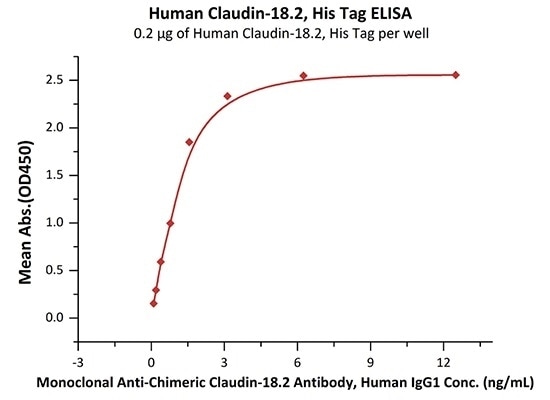
Immobilized Human Claudin-18.2, His Tag (Cat. No. CL2-H5546) at 2 μg/mL (100 μL/well) on a Nickel Coated plate can bind Monoclonal Anti-Chimeric Claudin-18.2 Antibody, Human IgG1 with a linear range of 0.4–2 ng/mL (in presence of DDM and CHS). Image Credit: ACROBiosystems
High affinity validated by SPR
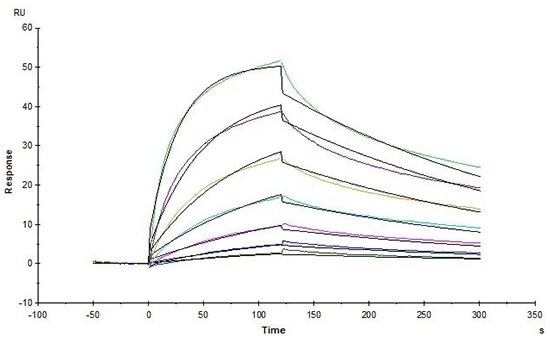
Human Claudin-18.2, His Tag (Cat. No. CL2-H5546) captured on CM5 chip via anti-His antibody can bind Monoclonal Anti-Chimeric Claudin-18.2 Antibody, Human IgG1 with an affinity constant of 3.55 nM as determined in an SPR assay (in presence of DDM and CHS) (Biacore T200). Image Credit: ACROBiosystems
High affinity validated by BLI
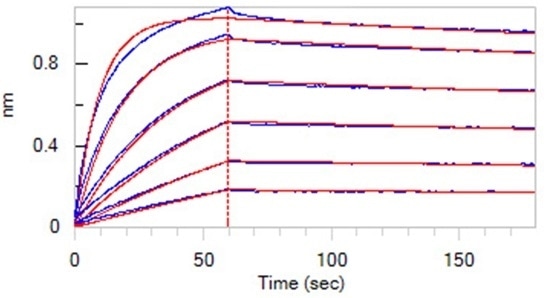
Loaded Monoclonal Anti-Chimeric Claudin-18.2 Antibody, Human IgG1 on AHC Biosensor, can bind Human Claudin-18.2, His Tag (Cat. No. CL2-H5546) with an affinity constant of 1.55 nM as determined in BLI assay (ForteBio Octet Red96e). Image Credit: ACROBiosystems.
High batch consistency validated by ELISA
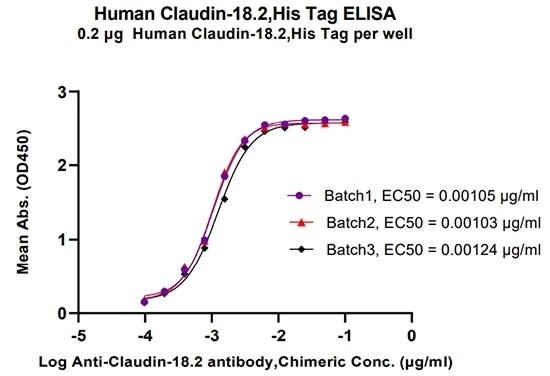
Human Claudin-18.2, His Tag (Cat. No. CL2-H5546) batch consistency (validated by ELISA) (CV < 10%). Image Credit: ACROBiosystems
Clinical drug information
Table 2. Source: ACROBiosystems
| ame |
Research Code |
Research Phase |
Company |
Indications |
Clinical Trials |
| Zolbetuximab |
GC-182; iMAB-362 |
Phase 3 Clinical |
Ganymed |
Lymphangioma, Cystic; Solid tumors; Stomach Neoplasms; Esophageal Neoplasms; Gastrointestinal Diseases; Pain; Pancreatic Neoplasms; Adenocarcinoma |
Details |
| Recombinant humanized anti-Claudin 18.2 monoclonal antibody (CARsgen) |
AB-011 |
Phase 1 Clinical |
Kaixing Life Technology (Shanghai) Co Ltd, Carsgen Biomedicine (Shanghai) Co Ltd |
Solid tumors; Stomach Neoplasms; Pancreatic Neoplasms |
Details |
| KD-022 (Nanjing KAEDI Biotech) |
KD-022; KD-182 |
Phase 1 Clinical |
Nanjing Kaedi Biotechnology Co Ltd |
Neoplasms |
Details |
| LCAR-C18S CAR-T cell therapy |
LB-1904; LB1904 |
Phase 1 Clinical |
Nanjing Legend Biotechnology Co Ltd |
Stomach Neoplasms |
Details |
Click here to learn more about clinical drug information.