Every year, a significant number of individuals across the globe contract the influenza virus, leading to the manifestation of severe symptoms and, in some cases, fatal outcomes, thereby posing a grave threat to human well-being. Vaccination prevents infections and reduces influenza-related mortality.
Given the limitations associated with current influenza vaccines, such as their reliance on fertilized chicken eggs, limited production capacity, the need for egg adaptation, and prolonged production time, exploring alternative methods is a must.
In light of these drawbacks, cell-based manufacturing presents itself as a viable alternative for the production of influenza vaccines.
The history of cell-based influenza vaccine development
Over the past 80 years, various methods have been developed to culture influenza viruses. In 1933, the first human influenza virus was isolated by propagating specimens obtained from throat washings collected from a patient, using ferrets through intranasal instillation.1
In 1935, Wilson Smith proposed a method that involved cultivating the influenza virus in the chorioallantoic membrane of embryonated eggs.2
This technique proved to be highly successful, yielding significantly higher concentrations of the virus compared to earlier methods that relied on extracting the virus from the lungs of infected animals.
Notably, fertilized chicken eggs continue to be employed in the present day for the production of influenza vaccines.
However, since the late 1950s, researchers have gradually transitioned towards utilizing various cell lines, such as Madin-Darby canine kidney (MDCK) and Vero cells, for the cultivation of influenza viruses. This shift from eggs to cells for influenza vaccine production. (Figure 1)
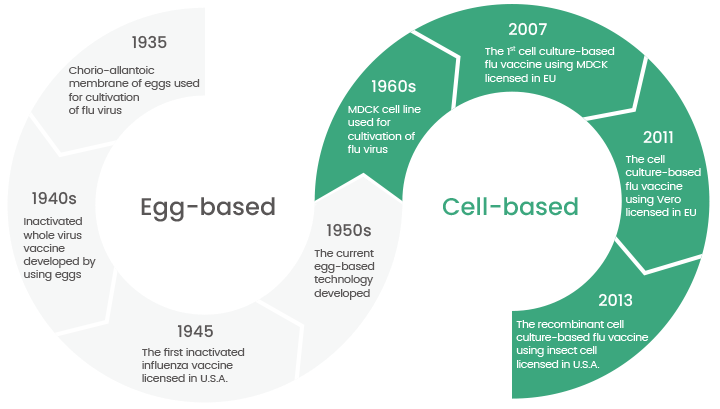
Figure 1. The transition from egg-based to cell-based flu vaccines is still in progress today. (Ref: DOI:10.1002/rmv.2243). Image Credit: Sino Biological Inc.
Cell-based vs. Egg-based influenza vaccines
For over 70 years, the primary method of producing influenza vaccines has relied on egg-based techniques. However, despite the extensive industrial manufacturing experience and safety regulatory filings, certain challenges have emerged in this process.
Inherent limitations in the manufacturing procedure involve the serial passage of the virus in chicken eggs, particularly with H3N2, which can result in egg-adaptive mutations affecting the antigenic sites and diminishing vaccine effectiveness.
Moreover, surges in the demand for chicken eggs, particularly during pandemics, hinder the timely production of vaccines. The occurrences of avian influenzas, notably the highly pathogenic avian influenza (HPAI) A/H5N1 outbreaks from 1997 to 2008, prompted researchers to explore cell-based vaccines as an alternative.
Additional disadvantages of the egg-based approach include a higher risk of allantoic fluid contamination, labor-intensive manufacturing systems, and potential allergic reactions among certain recipients. (Table 1)
To address these limitations, the adoption of culture-based vaccine production methods becomes crucial for the future success of the influenza vaccine industry.
Table 1. Comparison between cell- and egg-based influenza vaccines. Source: Sino Biological Inc.
| Items |
Egg-based flu vaccines |
Cell culture-based flu vaccines |
| Flu vaccine production technology |
The platform relies on a steady supply of embryonated eggs. However, supply can be overwhelmed by sudden increases in demand, such as during a pandemic. |
Viral production in a cell culture bioreactor is more flexible and scalable and is unaffected by egg shortages. |
| Production cycle |
6~8 Months. |
3 Months. |
| Passage adaptation variation |
Adaptive mutations may accumulate and potentially change the strain’s antigenicity. |
Low mutation rates and the antigenicity of the vaccine are closer to the wild type. |
| Allergic reaction |
May cause allergic reactions to ovalbumin. |
Ovalbumin-free to reduce the risk of post-vaccination anaphylaxis. |
| Influenza vaccine contamination |
The allantoic fluid of individual chicken embryos is contaminated, resulting in a high risk of contamination of the entire batch. |
Low risk of contamination when using closed systems such as cell engineering, microcarrier culture, and bioreactors. |
| Scale-up and automation |
Increased production is more limited and requires higher labor costs. |
A high degree of automation, large-scale cultivation, and easy scale-up. |
| Efficacy |
Not obvious. |
Not obvious. |
Cell-based influenza vaccines necessitate a more precise characterization and definition of raw materials and substrates compared to the production of egg-based flu vaccines. However, the scalable nature of the process grants increased flexibility in addressing heightened demand.
The World Health Organization (WHO) recommends the utilization of MDCK and Vero cells for the manufacture of influenza vaccines.
MDCK cells, an adherent cultured cell line, were originally derived from canine kidney tissue by Madin and Darby in 1958.3 In comparison to other cell lines, MDCK cells exhibit a higher rate of influenza virus replication and can be adapted to generate substantial viral titers in as few as 3–10 passages.
This shortens the production time of vaccine strains, and reducing the number of adaptive generations can also reduce the antigenic drift of the genes encoding the hemagglutinin (HA) and neuraminidase (NA) proteins that occurs during the passage of vaccine strains.
Thus, MDCK cells are now classed as a vital cell line suitable for influenza virus strain isolation and vaccine production. Furthermore, MDCK cells can be readily adapted to serum-free medium and serum-free suspension, facilitating large-scale industrial production.
Vero cells, as recommended by the WHO, serve as the designated cell line for vaccine production. In 2010, Baxter's Preflucel, a trivalent inactivated influenza vaccine, received approval from the EMA.4
This vaccine was produced using a Vero cell serum-free adherent culture. Studies have indicated that Vero cells also exhibit broad sensitivity to various types and subtypes of influenza viruses. However, their efficiency in virus amplification is lower compared to MDCK cells and chicken embryos.
Consequently, the yield of Vero cell-based influenza vaccines is relatively limited, necessitating research and development efforts to cultivate high-yielding influenza virus strains in Vero cells.
Challenges to cell-based influenza vaccines
The initial stage of cell-based vaccine production necessitates substantial R&D investment, which in turn leads to increased costs for patients. Biopharmaceutical companies transitioning to cell-based pipelines must consider two crucial aspects: vaccine safety and effectiveness.
One key consideration is the ability of a cell line to consistently replicate a diverse range of virus strains, thereby ensuring a high level of performance. Influenza viruses derived from cell cultures exhibit a greater resemblance to primary human virus strains, eliciting a more robust protective response.
However, cell-based production platforms are susceptible to variations stemming from host cell lines and culture conditions, which can impact their reliability.
To support the development of influenza vaccines, Sino Biological offers a comprehensive range of reagent products within its ProVir® viral antigen collection. This collection encompasses WHO-recommended vaccine strains of the HA, NA, and nucleoprotein (NP) proteins from recent years.
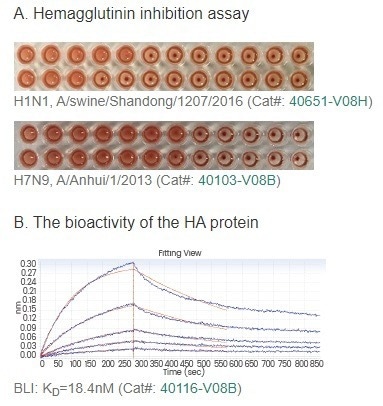
Furthermore, Sino Biological has developed an extensive assortment of monoclonal antibodies targeting flu antigens. These reagents prove invaluable in facilitating the development of relevant assays. (Figure 2)
C. Neutralizing antibodies
| Cat# |
Antibody type |
Application |
Antigen |
| 11055-MM11 |
Mouse MAb |
ELISA |
H1N1 HA |
| 11082-R019 |
Rabbit MAb |
ELISA,HI |
H7N7 HA |
| 11048-MM01 |
Mouse MAb |
WB, ELISA |
H5N1 HA |
| 68031-H011 |
Human Mab |
MN, HI |
H5N1 HA |
| 40359-M001 |
Mouse MAb |
MN, HI |
H10N8 HA |
| 40359-mh001 |
ChimericMAb |
MN, HI |
H10N8 HA |
Figure 2. The bioactivity of HA proteins is shown in the HA assay (A) and binding assay (B). Sino Biological provides neutralizing antibody products to meet various needs (C). Abbreviations: MN, Microneutralization; HI, Hemagglutinin Inhibition. Image Credit: Sino Biological Inc.
Licensed cell-based influenza vaccines
Significant advancements have been achieved in recent years regarding the R&D and industrialization of cell-based influenza vaccines. In 2001, the Sekirus Company (United States) successfully developed the Flucelvax® Quadrivalent (Flucelvax® TETRA) influenza vaccine.
This subunit vaccine is manufactured using MDCK cells that are cultured in adherence to serum-free microcarriers.
Flucelvax® Quadrivalent received its initial license in the United States in 2012 and is presently authorized for use in children aged four years and above.
Similarly, in 2007, the European Union approved Novartis' Optaflu, a trivalent subunit vaccine produced using the MDCK 33016 cell line cultured in a serum-free suspension.
In 2016, the FDA approved Flucelvax® Quadrivalent, another quadrivalent influenza subunit vaccine manufactured utilizing serum-free suspension MDCK cells, developed by Seqirus.
In South Korea, SK Chemicals has produced the SKYCellflu trivalent and quadrivalent subunit vaccines using MDCK cells. These vaccines have obtained licenses for use in children aged six months and older in Korea, and three years and older in Malaysia and Thailand.5
All these vaccines use MDCK cells or Vero cells as substrates. A summary of seasonal influenza vaccines is shown in Table 2.
Table 2. Cell-based seasonal influenza vaccine information. Source: Sino Biological Inc.
| Manufacturer |
Types of flu vaccines |
Cell type |
Cell culture method |
Approval |
| Solvay |
Trivalent inactivated subunit influenza vaccine (ccIIV3) |
MDCK |
Serum-free microcarrier culture |
Netherlands, 2001 |
| Baxter |
Trivalent inactivated whole virion influenza vaccine (ccIIV3) |
Vero |
Serum-free microcarrier culture |
Austria, 2010; EMA, 2011 |
| Novartis |
Trivalent inactivated subunit influenza vaccine (ccIIV3) |
MDCK |
Serum-free suspension culture |
EMA,2007; FDA,2012 |
| Seqirus |
Quadrivalent inactivated subunit influenza vaccine (ccIIV4) |
MDCK |
Serum-free suspension culture |
FDA,2016; EMA,2019 |
| SK Chemicals |
Quadrivalent inactivated subunit influenza vaccine (ccIIV4) |
MDCK |
Serum-free suspension culture |
Korea, 2015 |
Summary
Despite considerable advances in human vaccine manufacturing methods since the discovery of the influenza virus about 80 years ago, influenza remains a substantial burden on both human and animal populations.
MDCK cells have been widely used by scientific research organizations and laboratories across the world for decades.
MDCK cells are also used as a passage cell line for vaccine production, and their potential tumorigenicity and the risk of contamination by exogenous factors have become a widespread concern.
With the improvement of cell-based vaccines and the advancement of influenza vaccine production technology, it is only a matter of time before cell-based influenza vaccines completely replace chicken egg-based vaccines.
References and further reading
- Smith W et al. A virus obtained from influenza patients. Lancet. DOI: 10.1016/S0140-6736(00)78541-2
- Eisfeld A. J., et al. Influenza A virus isolation, culture and identification. Nature protocols. DOI: 10.1038/nprot.2014.180
- Pérez Rubio A et al. Cell culture-derived flu vaccine: Present and future. Hum Vaccin Immunother. DOI: 10.1080/21645515.2018.1460297.
- https://www.ema.europa.eu/en/medicines/human/referrals/preflucel
- Rockman S et al. New Technologies for Influenza Vaccines. Microorganisms. DOI:10.3390/microorganisms8111745.
About Sino Biological Inc.

Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offers pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.