Antibody-drug conjugates (ADCs) are a growing class of biotherapeutics that have transformed targeted therapy, especially in oncology. By making use of monoclonal antibodies’ specificity to deliver potent cytotoxic drugs to cancer cells directly, ADCs can selectively eradicate cancer cells with fewer off-target effects.1
While traditional ADCs have some success, they are frequently limited in terms of efficacy, safety, and flexibility. Bispecific ADCs or multi-specific ADCs, alongside other innovative designs, seek to overcome these hurdles by integrating extra targeting mechanisms and functionality.
Beyond novel ADC designs, it is notable that novel ADC use cases in domains beyond conventional oncology, including autoimmune conditions, infectious conditions, and other chronic conditions, underscores upcoming trends of ADC development.
Novel and promising designs of ADC - Bispecific ADCs (BsADCs)
The main source of ADC toxicity is the non-specific binding of antibodies to antigens.2 Improving the selectivity and specificity of ADCs can greatly diminish toxicity and increase their potential use cases. One innovative solution to this challenge is the development of BsADCs, which implicates the conjugation of bispecific antibodies (BsAbs) to linker‒payload complex.3
Using two distinct antigen-binding sites within a single molecule, BsADCs could increase specificity and diminish the likelihood of off-target effects by recognizing dual targets that manifest on tumor cells (Fig 1).
This dual specificity is especially useful for heterogeneous tumors or conditions where pathogenic cells express different markers. By engaging two antigens, BsADCs can additionally enhance binding avidity and boost the likelihood of target cell internalization, thus improving payload delivery. 4
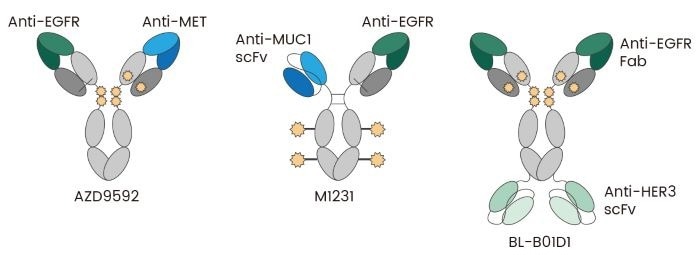
Fig 1. Bispecific ADC Examples: AZD9592 recognizes EGFR and MET; M1231 recognizes EGFR and MUC1; BL-B01D1 recognizes EGFR and HER3 5. Image Credit: Sino Biological Inc.
An example of this is the BsADC design targeting HER2×CD63. As HER2's resistance to internalization may reduce drug efficacy, BsADC targeting HER2×CD63 successfully improves the effective internalization of HER2 and assists lysosomal co-localization.6
BsADCs are built to detect both dual antigens and two unique epitopes of the same antigen (known as biparatopic ADCs), which expands their range of potential use cases. For example, ADC ZW49, which distinguishes HER2 ECD4 and ECD2 at the same time, can aid receptor clustering, internalization, and lysosomal trafficking (Fig 2). It has shown both a manageable safety profile and promising antitumor activity.7
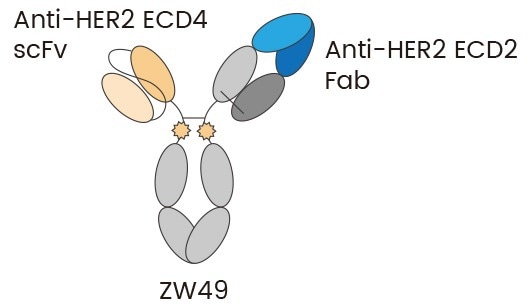
Fig 2. ZW49 is a heterodimeric ADC capable of bivalent binding to HER2 ECD2 and ECD4 5. Image Credit: Sino Biological Inc.
Bispecific ADCs enables promising therapeutic opportunities through their targeting of a wider spectrum of antigens, improving efficacy, and potentially enhancing safety profiles. Yet, this format also comes with hurdles that require careful consideration.
For example, a risk of unintended receptor activation exists, as reported with anti-MET ADC (d) and EGFR–MET-directed ADC.8 Moreover, disparities in the expression levels of the two target antigens between tumors and patients may complicate the identification of optimal responders. A comprehensive evaluation of epitopes, binding mechanisms, and the underlying biology is thus crucial to guarantee optimal treatment results.
ADCs beyond oncology: Targeting autoimmune diseases
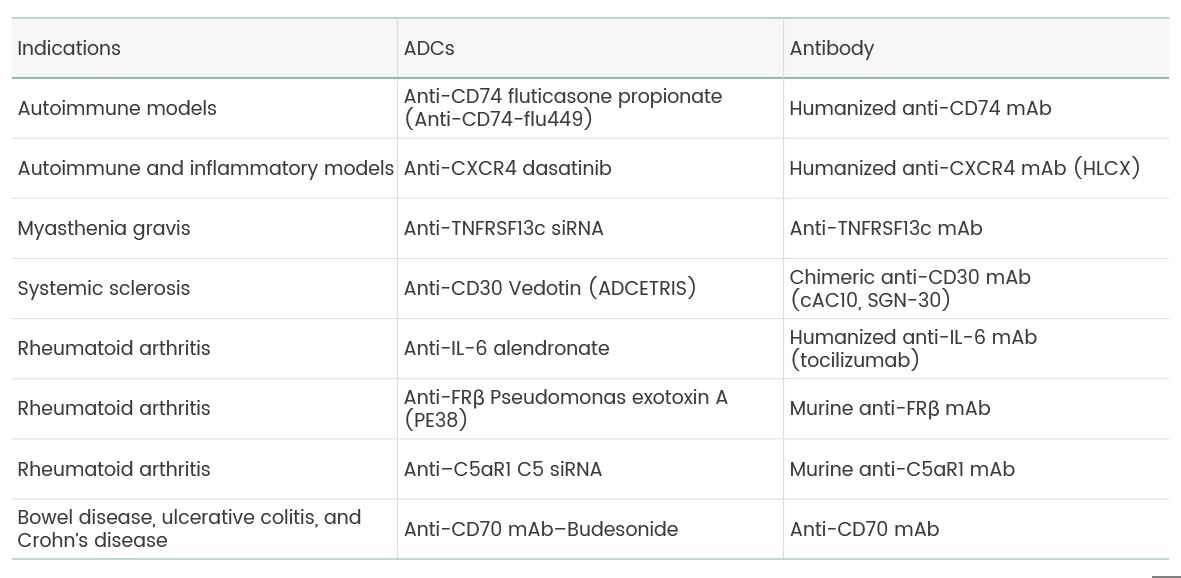
Although ADCs have mostly been developed for cancer therapy, their mechanism of action is also applicable for other non-oncological diseases, such as atherosclerosis, muscular conditions, and infectious conditions, especially autoimmune conditions. Many efforts have been made to explore the potential of ADCs in multiple autoimmune disorders, including rheumatoid arthritis, myasthenia gravis, systemic sclerosis, and ulcerative colitis (Table 1).9
Table 1. Antibody-Drug Conjugates (ADCs) and Their Indications. Source: Sino Biological Inc.
Autoimmune diseases occur from a dysfunctional immune response where the body erroneously attacks its own tissues. Conventional therapies typically involve systemic immunosuppression, which can result in significant side effects and higher susceptibility to infections.10
ADCs, with their precision targeting, provide a different approach by selectively depleting pathogenic immune cells or modulating specific signaling pathways. A key example is the use of ADCs targeting CD19, a marker found on B cells.11 B-cell depletion is a therapeutic strategy for conditions including systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA).
Upcoming ADCs, including those coupling anti-CD19 antibodies with immunosuppressive payloads, show promise in preclinical research for targeted B-cell depletion while leaving healthy tissues unaffected. 12 For example, anti-CD19 ADCs are currently being evaluated for their safety and efficacy, and early results suggest significant reductions in disease activity.13
A further example is that CD6-ADC has demonstrated promise in treating two preclinical models of autoimmune uveitis and a humanized model of GVHD, indicating that CD6-ADC may be developed even further as a therapeutic agent to selectively eradicate autoreactive T cells, and as a promising way to treat several other T cell–mediated conditions.14
Anti-TNF ADC was additionally built to potentially treat rheumatoid arthritis, which is a chronic, systemic autoimmune condition.9
One of the biggest issues in using ADCs to treat autoimmune conditions lies in selecting appropriate targets. Autoimmune conditions are typically systemic, and identifying specific markers that are expressed only on pathogenic cells is crucial for avoiding off-target effects. Moreover, the immunomodulatory payloads utilized in these ADCs should balance efficacy with safety to stave off unintended suppression of the wider immune system.9
The clinical development of ADCs in autoimmune conditions additionally faces challenges including optimizing dosing regimens and reducing immunogenicity. Dissimilar to oncology, where rapid tumor cell killing is frequently desired, autoimmune diseases may demand more subtle modulation of immune pathways, necessitating careful preclinical and clinical study designs.
Future perspectives
The growing scope of ADC drugs carries great potential for meeting unaddressed medical needs. As ADCs transition into non-oncology indications and adopt novel designs such as bispecific ADCs, they are set to become a critical part of precision medicine.
However, many hurdles remain, such as improving target selection, enhancing manufacturability, and guaranteeing cost-effectiveness to improve accessibility. Upcoming trends in ADC therapeutics, including their use in autoimmune conditions and the dawn of bispecific designs, are revolutionizing the landscape of targeted therapy. These innovations highlight the flexibility of ADCs and potential to treat complex conditions with precision and efficacy.
Sino Biological's efforts on new trends of ADC development
Sino Biological stands at the forefront of advancements in ADC research with a complete suite of products and services. The company offers outstanding ADC development solutions that cover the journey from early discovery to clinical study to fully support clients during the ADC development.
Specializing in recombinant protein production, Sino Biological has built numerous high-quality ADC target protein products with the choice of several species and labels, that include well-established targets like HER-2, TROP-2, Nectin-4, EGFR, CD19, and BCMA, and upcoming targets such as EphA3, GFRA1, CLEC7A.
Sino Biological additionally offers a fast and efficient bispecific antibody production service alongside a broad spectrum of target proteins for autoimmune conditions that address the needs for ADC future directions on new designs and novel applications alike.
References and further reading
- Beck, A., et al. (2017). Strategies and challenges for the next generation of antibody–drug conjugates. Nature Reviews Drug Discovery, 16(5), pp.315–337. https://doi.org/10.1038/nrd.2016.268.
- Tarantino, P., et al. (2023). Optimizing the safety of antibody–drug conjugates for patients with solid tumours. Nature Reviews Clinical Oncology, 20(8), pp.558–576. https://doi.org/10.1038/s41571-023-00783-w.
- Gu, Y., Wang, Z. and Wang, Y. (2024). Bispecific antibody drug conjugates: Making 1+1>2. Acta Pharmaceutica Sinica B. (online) https://doi.org/10.1016/j.apsb.2024.01.009.
- Dumontet, C., et al. (2023). Antibody–drug conjugates come of age in oncology. Nature Reviews Drug Discovery, (online) 22(8), pp.641–661. https://doi.org/10.1038/s41573-023-00709-2.
- Tsuchikama, K., et al. (2024). Exploring the next generation of antibody–drug conjugates. Nature Reviews Clinical Oncology, (online) pp.1–21. https://doi.org/10.1038/s41571-023-00850-2.
- de Goeij, B.E., et al. (2014). HER2 monoclonal antibodies that do not interfere with receptor heterodimerization-mediated signaling induce effective internalization and represent valuable components for rational antibody-drug conjugate design. mAbs, 6(2), pp.392–402. https://doi.org/10.4161/mabs.27705.
- Hamblett, K., et al. (2019). Abstract P6-17-13: ZW49, a HER2 targeted biparatopic antibody drug conjugate for the treatment of HER2 expressing cancers. Cancer Research, 79(4_Supplement), pp.P6-1713-P617–13. https://doi.org/10.1158/1538-7445.sabcs18-p6-17-13.
- Neijssen, J., et al. (2021). Discovery of amivantamab (JNJ-61186372), a bispecific antibody targeting EGFR and MET. Journal of Biological Chemistry, 296, p.100641. https://doi.org/10.1016/j.jbc.2021.100641.
- Lal Bahadur Pal, Bule, P., Khan, W. and Naveen Chella (2023). An Overview of the Development and Preclinical Evaluation of Antibody–Drug Conjugates for Non-Oncological Applications. Pharmaceutics, 15(7), pp.1807–1807. https://doi.org/10.3390/pharmaceutics15071807.
- Ruiz, R. and Kirk, A.D. (2015). Long-Term Toxicity of Immunosuppressive Therapy. Transplantation of the Liver, pp.1354–1363. https://doi.org/10.1016/b978-1-4557-0268-8.00097-x.
- Andy Qingan Yuan, Yang, I., et al. (2024). Targeting CD19 for the treatment of B-cell related autoimmune diseases with a novel T cell engager. bioRxiv (Cold Spring Harbor Laboratory). https://doi.org/10.1101/2024.02.17.580750.
- Carter, P.J. and Lazar, G.A. (2017). Next generation antibody drugs: pursuit of the ‘high-hanging fruit’. Nature Reviews Drug Discovery, 17(3), pp.197–223. https://doi.org/10.1038/nrd.2017.227.
- Peng, B.J., et al. (2024). Preclinical specificity and activity of a fully human 4-1BB expressing anti-CD19 CART therapy for treatment-resistant autoimmune disease. Molecular therapy. Methods & clinical development, 32(2), pp.101267–101267. https://doi.org/10.1016/j.omtm.2024.101267.
- Zhang, L., et al. (2023). A CD6-targeted antibody-drug conjugate as a potential therapy for T cell-mediated disorders. JCI insight, (online) 8(23), p.e172914. https://doi.org/10.1172/jci.insight.172914.
About Sino Biological Inc.
Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.