A revolution has occurred in the field of pharmaceutical development due to the development of drugs that target membrane proteins. It is estimated that one-third of currently available drugs target G protein-coupled receptors (GPCRs).
Image Credit: Sino Biological Inc.
Further development, however, is restricted because of the complex structures, low expression levels, in vitro insolubility, instability during purification, and challenges in the preservation of the native conformation of these proteins.
Addressing these issues demands a mixture of specialized expertise, optimization strategies, and advanced purification methods.
What are transmembrane proteins?
Approximately one-third of all known sequence-encoded proteins are membrane proteins. These proteins play critical roles in many cellular processes, such as energy utilization, molecular transport, signal transduction, and the maintenance of membrane homeostasis.1-3
Membrane proteins may be classified into three types based on structure; these types are lipid-anchored, peripheral, and integral.
Lipid-anchored membrane proteins are covalently attached to the bilayer by lipid moieties and specific enzymes are required for them to be released, while peripheral membrane proteins can be easily dissociated without any disruption to the membrane structure.
Integral membrane proteins are firmly embedded within the lipid bilayer and the membrane structure must be disrupted to remove them.4-5
These proteins carry out many functions, such as acting as G protein-coupled receptors (GPCRs), transport proteins, ion channels, and other types of membrane receptors.
GPCRs are highly attractive targets for drug screening due to their abundance in eukaryotes, where they interact with external molecules to trigger intracellular signaling.
Ion channels play a crucial role in the maintenance of cellular integrity as they regulate the levels of ions such as potassium, sodium, and calcium.
Transport proteins enable molecules and ions to move across biological membranes for the regulation of metabolic processes within organisms. At present, approximately 60% of drug targets are membrane. 6
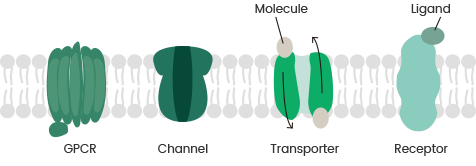
Image Credit: Sino Biological Inc.
Challenges for membrane protein expression:
Although there is great potential for membrane proteins in drug development, the recombinant expression of these proteins poses a challenge in the realization of this potential for the following reasons:
- Membrane localization: Transmembrane proteins are integrated into cellular membranes, which can increase the complexity of their expression compared to soluble proteins. This is because the correct targeting and insertion into the membrane is difficult, as is maintaining native conformation.7
- Hydrophobicity: Transmembrane proteins have large hydrophobic regions that span the lipid bilayer to anchor them in the membrane. This can lead to issues for protein expression in aqueous environments, such as aggregation, misfolding, or degradation.8
- Protein toxicity: The overexpression of transmembrane proteins, particularly those with high hydrophobicity, may be toxic to the host cell; the accumulation of misfolded or unfolded proteins in the endoplasmic reticulum can trigger cellular stress responses, including the unfolded protein response, resulting in cell death.9,10
- Membrane integration and folding: Transmembrane proteins demand certain chaperones and translocation machinery, as well as other cellular factors for the correct folding and membrane insertion. The malfunction or lack of these factors can lead to inefficient membrane integration or misfolding of the protein.7
- Structural complexity: Transmembrane proteins typically have complex structures with multiple loops, domains, and transmembrane segments. This can impact the successful expression and purification, and make the structural characterization more difficult.8
- Low expression levels: Some transmembrane proteins have naturally low expression levels, which can cause challenges for detection and purification. Limited amounts of protein can negatively impact downstream functional and structural studies.11-13
Sino Biological offers three multi-pass transmembrane protein development platforms
Sino Biological is dedicated to delivering a full range of custom recombinant protein expression services. The company focuses on all aspects of protein study, including expression, purification, characterization, and stabilization.
Sino Biological is the market leader in high-quality multi-pass transmembrane protein products. The company has created three HEK293-expression-based platforms to offer premium raw materials for early-stage drug development. These platforms include detergent micelles, virus-like particles (VLP), and nanodiscs.
1) Virus-like particle platform
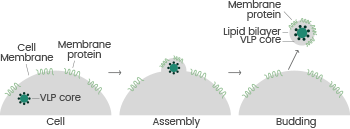
Image Credit: Sino Biological Inc.
Sino Biological employs the VLP technology platform (based on the HEK293 mammalian expression system), which utilizes nano-scale, virus-derived self-assembling structures to enable full-length transmembrane target proteins to be displayed on the VLP surface.14-15
This allows expressed transmembrane proteins to be detected in their native state, which is ideal for immunization and antibody screening purposes.
Using this platform, it has been found that membrane proteins such as 4-pass (Claudin-6, Claudin-9, Claudin-18.1, Claudin-18.2) and 7-pass transmembrane proteins (CXCR4, GPRC5D, and SSTR2) have high activity levels after expression.
2) Detergent micelle platform
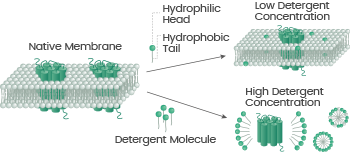
Image Credit: Sino Biological Inc.
The detergent micelle technology platform guarantees the production of membrane protein products that are optimized for immunization, SPR/BLI assays, ELISA, and more.
It does this by applying a conventional technique for membrane protein extraction, which involves surface-active compounds to form non-covalent conformation-protected clusters in solution because of the hydrophobic effect.16, 17
3) Nanodisc platform
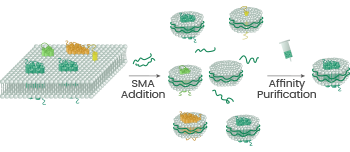
Image Credit: Sino Biological Inc.
The nanodisc platform utilizes the fact that transmembrane proteins preserve their functionality when attached to phospholipids to create stable products, which are ideal for applications including antibody screening and immunization.
The synthetic SMA polymer inserts into the cell membrane to “cookie-cut” the protein from the membrane while maintaining the natural conformation and activity of the protein.18-19
Featured GPRC5D transmembrane proteins
GPRC5D is a 7-pass transmembrane protein with a highly complex structure. This presents significant challenges for antibody development, antigen preparation, and activity analysis.
Using three systems (detergent, VLP, and nanodisc), Sino Biological has successfully developed GPRC5D transmembrane protein products, which were all expressed in HEK293.
Using SPR, ELISA, and BLI, these products were verified and found to have high activity and batch-to-batch consistency. This makes them sufficient for various applications, including CAR-positivity rate detection, animal immunity, and cell experiments, which fully support the development of GPRC5D drugs.
Human GPRC5D-VLP (Full length) protein | Cat#: 24447-HNAH
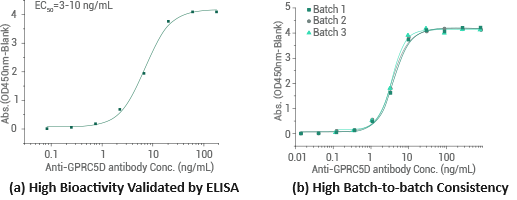
Graphs illustrating (a) the binding of immobilized recombinant Human GPRC5D-VLP (full length) protein at 5 μg/mL (100 μL/well) binding to anti-GPRC5D antibody and (b) the binding of proteins from different batches exhibiting minimal batch-to-batch variability. Image Credit: Sino Biological Inc.
Human GPRC5D-Detergent protein (His & FLAG Tag) | Cat#: 24447-H18H-DD
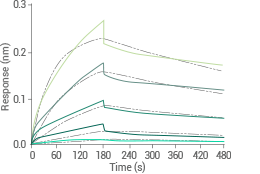
Anti-GPRC5D antibody on Protein A Biosensor binds the Human GPRC5D-Detergent Protein (His & FLAG Tag) with an affinity constant of 58.1 nM, as determined by BLI assay (ForteBio Octet Red384). Image Credit: Sino Biological Inc.
Human GPRC5D-Nanodisc protein (His and FLAG Tag) | Cat#: 24447-H18H-NA
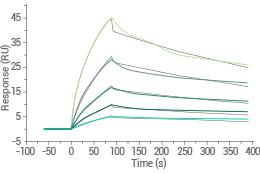
Captured anti-GPRC5D antibody on anti-Human IgG Fc via CM5 Chip binds the Human GPRC5D-Nanodisc Protein (His & FLAG Tag) with an affinity constant of 9.609 nM, as determined by SPR (Biacore T200). Image Credit: Sino Biological Inc.
Additionally, Sino Biological provides custom services and multi-pass transmembrane protein production using all three platforms to assist drug-discovery approaches.
Advantages of VLP, detergent micelle, and SMA-nanodisc platforms
Source: Sino Biological Inc.
| Platform |
Advantages |
| VLP Platform |
- Delivered within three to four weeks
- VLPs display membrane proteins on the native membrane with their natural conformation
- High immunogenicity
- Greater abundance of target proteins compared to cell immunization products
- Applications include ELISA, immunization, and CAR detection
|
| Detergent Micelle Platform |
- Pure product as the target membrane protein only
- The target protein can be quantified accurately
- Micelles offer hydrophobic protection for solubilized membrane proteins, allowing them to maintain their conformation
- Applications include ELISA, immunization, ELISA, and SPR/BLI
|
| SMA-Nanodisc Platform |
- A membrane protein is encapsulated by the SMA polymer in the native bilayer environment, maintaining the natural conformation and high activity
- Pure product as the target membrane protein without MSP
- High stability
- The target protein can be quantified accurately
- Applications include ELISA, immunization, cell experiments, SPR/BLI, and CAR detection
|
Sino Biological is continuing to grow its product list of transmembrane proteins, with the addition of more members of the Claudin and GPCR families as well as different immune receptors. The company is keen to be involved in custom, collaborative projects for the expression and production of new targets.
References
- Levental, I. and Lyman, E. (2023) Regulation of membrane protein structure and function by their lipid nano-environment. Nat. Rev. Mol. Cell Biol. 24, 107–122 https://doi.org/10.1038/s41580-022-00524-4
- Piper, S.J., Johnson, R.M., Wootten, D. and Sexton, P.M. (2022) Membranes under the magnetic lens: a dive into the diverse world of membrane protein structures using Cryo-EM. Chem. Rev. 122, 13989–14017 https://doi.org/10.1021/acs.chemrev.1c00837
- Autzen, H.E., Julius, D. and Cheng, Y. (2019) Membrane mimetic systems in CryoEM: keeping membrane proteins in their native environment. Curr. Opin. Struct. Biol. 58, 259–268 https://doi.org/10.1016/j.sbi.2019.05.022
- White, S. H. (2009). How membranes shape protein structure. Journal of Biological Chemistry, 284(14), 9037-9041.
- Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., & Walter, P. (2002). Molecular Biology of the Cell (4th ed.). Garland Science.
- Overington, J.P., Al-Lazikani, B. and Hopkins, A.L. (2006) How many drug targets are there? Nat. Rev. Drug Discov. 5, 993–996 https://doi.org/10.1038/nrd2199
- von Heijne, G. (2007). Membrane-protein topology. Nature Reviews Molecular Cell Biology, 7(12), 909-918.
- Vinothkumar, K. R., & Henderson, R. (2016). Structures of membrane proteins. Quarterly Reviews of Biophysics, 49, e13.
- Wagner, S., Klepsch, M. M., Schlegel, S., Appel, A., Draheim, R., Tarry, M., ... & De Gier, J. W. (2008). Tuning Escherichia coli for membrane protein overexpression. Proceedings of the National Academy of Sciences, 105(38), 14371-14376.
- Drew, D., Newstead, S., Sonoda, Y., Kim, H., & von Heijne, G. (2008). GFP-based optimization scheme for the overexpression and purification of eukaryotic membrane proteins in Saccharomyces cerevisiae. Nature Protocols, 3(5), 784-798.
- Drew, D., & Sjöstrand, D. (2013). Overcoming bottlenecks in membrane protein structural biology. Current Opinion in Structural Biology, 23(5), 919-925.
- Granseth, E., & von Heijne, G. (2007). Elofsson A. A study of the membrane-water interface region of membrane proteins. Journal of Molecular Biology, 367(1), 1071-1082.
- Hunte, C., & Michel, H. (2002). Crystallization of membrane proteins mediated by antibody fragments. Current Opinion in Structural Biology, 12(4), 503-508.
- Naskalska A, Pyrć K. Virus like particles as immunogens and universal nanocarriers[J]. Polish journal of microbiology, 2015, 64(1).
- Nooraei S, Bahrulolum H, Hoseini Z S, et al. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers[J]. Journal of nanobiotechnology, 2021, 19(1): 1-27.
- Newstead, S., Ferrandon, S., Iwata, S., & Byrne, B. (2008). Incoming substrate trajectory in the glutamate transporter homologue GltPh. Nature Structural & Molecular Biology, 15(4), 380-387.
- Wang, W., & Tajkhorshid, E. (2010). Electrostatic funneling of substrate in mitochondrial inner membrane carriers. Proceedings of the National Academy of Sciences, 107(13), 633-638.
- Unger, L., Ronco-Campaña, A., Kitchen, P., Bill, R.M. and Rothnie, A.J. (2021) Biological insights from SMA-extracted proteins. Biochem. Soc. Trans. 49, 1349–1359
- Dörr, J.M., Koorengevel, M.C., Schäfer, M., Prokofyev, A.V., Scheidelaar, S., van der Cruijsen, E.A. et al (2014) Detergent-free isolation, characterization, and functional reconstitution of a tetrameric K+ channel: the power of native nanodiscs. Proc. Natl Acad.
About Sino Biological Inc.
Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.