While chemotherapy is a very commonly used cancer therapy, it frequently causes serious side effects.
Antibody-drug conjugates (ADCs) are a promising emerging cancer therapy that combines the effective killing power of small molecule cytotoxins and the highly specific targeting ability of monoclonal antibodies (mAbs).
As a result, ADCs accurately deliver cytotoxins to tumor cells with minimal effect on normal cells. ADCs are currently a popular area for anticancer drug development.1,2
Gemtuzumab ozogamicin is a first-generation ADC that gained approval from the U.S. Food and Drug Administration (FDA) in 2000. Many ADCs have been approved globally since then, with over 100 ADC candidates having entered the clinical phase. These novel anticancer drugs are paving the way for a new cancer therapy phase.
Table 1. Currently approved ADCs on the market. Source: Sino Biological Inc.
| Targets |
Drugs |
Approval |
Indications |
| CD33 |
Gemtuzumab ozogamicin |
2000-FDA; 2017-FDA |
Acute myeloid leukemia |
| CD30 |
Brentuximab vedotin |
2011-FDA |
Hodgkin lymphoma |
| HER2 |
Trastuzumab emtansine |
2013-FDA |
Breast cancer |
| CD22 |
Inotuzumab ozogamicin |
2017-FDA |
Acute myeloid leukemia |
| CD22 |
Moxetumomab pasudotox |
2018-FDA |
Hairy cell leukemia |
| CD79 |
Polatuzumab vedotin |
2019-FDA |
Diffuse large B-cell lymphoma |
| Nectin4 |
Enfortumab vedotin |
2019-FDA |
Advanced urothelial cancer |
| HER2 |
Trastuzumab deruxtecan |
2019-FDA |
Breast cancer |
| TROP2 |
Sacituzumab govitecan |
2020-FDA |
Triple-negative breast cancer |
| EGFR |
Cetuximab Saratolacan |
2020-MHLW |
Head and neck cancer |
| BCMA |
Belantamab mafodotin |
2020-FDA: Withdrawal in 2022 |
Multiple myeloma |
| CD19 |
Loncastuximab tesirine |
2021-FDA |
Large B-cell lymphoma |
| Tissue factor |
Tisotumab vedotin-tftv |
2021-FDA |
Cervical cancer |
| HER2 |
Disitamab vedotin |
2021-NMPA |
Gastric or gastroesophageal cancer |
| FRα |
Mirvetuximab soravtansine |
2022-FDA |
Ovarian Cancer |
Key components of ADCs
An ADC is comprised of a cytotoxic payload, a target-specific mAb, and a chemically synthesized linker that covalently links the antibody and the toxin. The mAb binds to specific antigens on the surface of tumor cells, and ADCs are internalized into tumor cells during the formation of antibody-antigen complexes.
ADCs are usually transported to the lysosome from the endosome, where the linker is divided, and the small molecule cytotoxins are released, resulting in tumor cell death.3 The target antigens, antibodies, cytotoxic payload, and linker need to be considered in ADC development (see Figure 1).
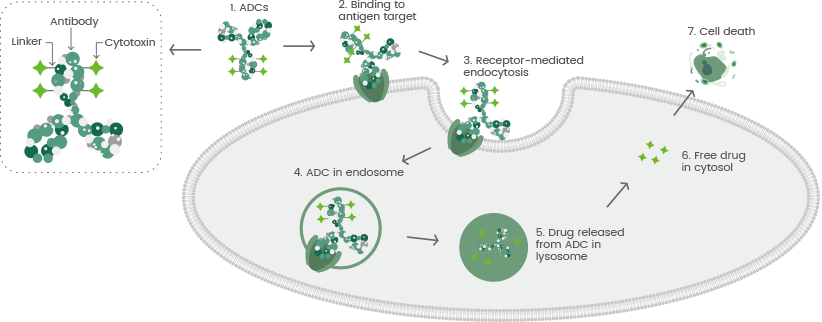
Figure 1. The general mechanism of action for antibody-drug conjugates (ADCs). Image Credit: Sino Biological Inc.
Target antigens
Target antigens should be non-secretory and expressed mainly on the tumor cell surface and at low levels in normal tissues.
Target antigens should also be internalized after binding to the corresponding antibodies to enable the ADC-antigen complexes to enter tumor cells for the release of cytotoxic payloads using a suitable intracellular translocation route.4
Currently, the target antigens for ADC drugs that have been approved are specific proteins that are overexpressed in tumor cells, such as Nectin4, HER2, EGFR, and Trop2 in solid tumors, and CD19, CD22, CD30, CD33, CD79, and BCMA in hematologic malignancies.
Emerging targets, including CD70, CD166, EpCAM, and CD25, are undergoing development and are already showing promising results.5
Sino Biological is leading the ADC therapy field, offering quality products for ADC target antigens.
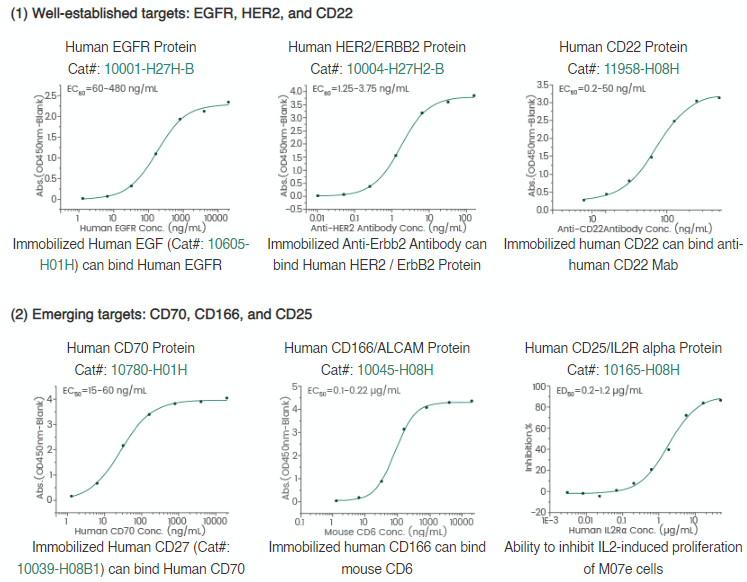
Figure 2. High-quality target antigen products. Image Credit: Sino Biological Inc.
Antibodies
The use of immunoglobulins (IgGs) and their derivatives is widespread in clinical studies. IgG1, IgG2, and IgG4 are mostly utilized for ADCs due to their specificity, long circulating half-life, and affinity for the target antigen. The antibodies should also be effective for internalization and have low immunogenicity.6
For early ADC drug development, mouse antibodies were predominantly utilized. However, these antibodies caused a significant immune response, which led to a reduction in therapeutic efficacy.
Since the introduction of recombinant technology, chimeric and humanized antibodies with considerably lower immunogenicity have replaced mouse antibodies.7
Sino Biological delivers high-quality mAb humanization services using computer-aided molecular modeling and complementarity-determining region (CDR) grafting technology.
Additionally, Sino Biological has the high-throughput and scale-up abilities needed for the production of highly efficient antibodies in CHO and HEK293 cells in as little as two weeks, with more than 1000 Abs expressions per batch.
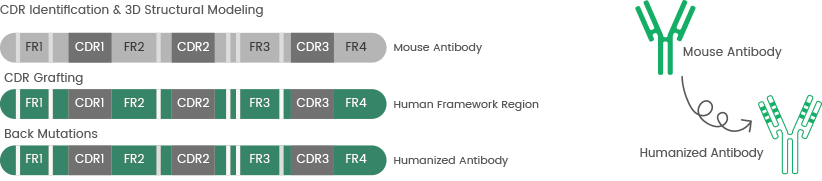
Figure 3. The process of antibody humanization and complementarity-determining region (CDR) grafting technology. Image Credit: Sino Biological Inc.
Cytotoxic payload
Cytotoxins should have high stability and toxicity and low immunogenicity. Additionally, the cytotoxin should have a modifiable functional group or a site where a functional group can be introduced to link the mAb.
The most frequently utilized cytotoxins for ADC drugs in clinical trials or on the market are microtubule protein inhibitors or DNA-damaging agents.8
Additionally, the drug-antibody ratio (DAR), i.e., the number of drug molecules attached to the antibody by the linker, is a key factor in ADC development. A low DAR may reduce ADC efficacy, while a high DAR may cause instability, leading to off-target toxicity.8,9
Linker
The linker ensures that, during circulation, the cytotoxic payload is securely attached to the antibody in the plasma to prevent premature release that would cause damage to normal cells or tissues and ensure the release within the target tumor cells is effective.
Linkers can be categorized as cleavable and non-cleavable. While cleavable linkers use the environmental differences between the circulation and tumor cells for the precise release of free cytotoxins, non-cleavable linkers depend on the lysosomal degradation of the entire antibody-linker structure.
This causes the retention of charged amino acids in the payload.10,11
Challenges and perspectives
There are still numerous challenges in ADC development. The most significant challenge is the toxic effects of ADCs, such as leukopenia, thrombocytopenia, neutropenia, anemia, and gastrointestinal effects.4
An additional challenge is the resistance of tumors ADCs, as demonstrated by decreased antigen expression levels, payload resistance, and changed intracellular transport pathways.
Another challenge is payload release. The ADCs are much greater than conventional cytotoxic drugs, and there is limited efficiency of cytotoxin penetration into tumors.
Future ADC development should focus on the following aspects to overcome the above challenges:
- Improvements in ADC design, including linkers, payload platforms, and coupling strategies, to minimize toxicity.
- Utilization of two cytotoxic agents as payloads to minimize ADC resistance.
- Improving lysosomal delivery and ADC internalization via bispecific antibodies to enhance antitumor specificity. Sino Biological uses an optimized mammalian cell expression platform to deliver rapid and efficient bispecific antibody expression services for clients worldwide.12
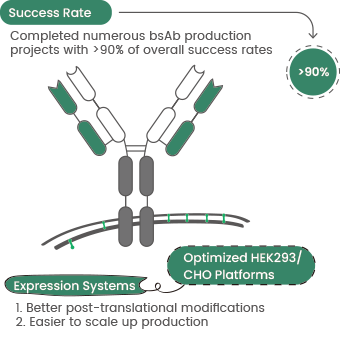
Figure 4. Sino Biological bispecific antibody production service highlights. Image Credit: Sino Biological Inc.
Many cancer patients have benefited from ADC therapy. In the future, the therapeutic efficacy of ADC will be improved by reducing ADC toxicity and drug resistance.
Sino Biological will continue to lead the ADC therapy field, delivering high-quality products and one-stop services to pharmaceutical companies and researchers.
References
- Fu Z, Li S, Han S, et al. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduction and Targeted Therapy. 2022;7(1):93. doi: 10.1038/s41392-022-00947-7
- Schwach J, Abdellatif M, Stengl A. More than Toxins—Current Prospects in Designing the Next Generation of Antibody Drug Conjugates. Frontiers in Bioscience-Landmark. 2022;27(8):240. doi: 10.31083/j.fbl2708240
- Tang H, Liu Y, Yu Z, et al. The analysis of key factors related to ADCs structural design. Frontiers in Pharmacology. 2019;10:373. doi: 10.3389/fphar.2019.00373
- Zhao P, Zhang Y, Li W, et al. Recent advances of antibody drug conjugates for clinical applications. Acta Pharmaceutica Sinica B. 2020;10(9):1589-1600. doi: 10.1016/j.apsb.2020.04.012
- Hafeez U, Parakh S, Gan HK, et al. Antibody–drug conjugates for cancer therapy. Molecules. 2020;25(20):4764. doi: 10.3390/molecules25204764
- Zhang X, Huang AC, Chen F, et al. Novel development strategies and challenges for anti-Her2 antibody-drug conjugates. Antibody Therapeutics, 2022;5(1):18-29. doi: 10.1093/abt/tbac001
- Khongorzul P, Ling CJ, Khan FU, et al. Antibody–Drug Conjugates: A Comprehensive ReviewAntibody–Drug Conjugates in Cancer Immunotherapy. Molecular Cancer Research. 2020;18(1):3-19. doi: 10.1158/1541-7786.MCR-19-0582
- Ziad A, Abdurahman A, Misako N. A comprehensive review on antibody-drug conjugates (ADCs) in the treatment landscape of non-small cell lung cancer (NSCLC). Cancer Treatment Reviews. 2022;102393. doi: 10.1016/j.ctrv.2022.102393
- Sheyi R, de la Torre BG, Albericio F. Linkers: An Assurance for Controlled Delivery of antibody-drug conjugate. Pharmaceutics. 2022;14(2):396. doi: 10.3390/pharmaceutics14020396
- Tong JTW, Harris PWR, Brimble MA, et al. An insight into FDA approved antibody-drug conjugates for cancer therapy. Molecules. 2021;26(19):5847. doi: 10.3390/molecules26195847
- Ungaro A, Tucci M, Audisio A, et al. Antibody-drug conjugates in urothelial carcinoma: a new therapeutic opportunity moves from bench to bedside. Cells. 2022;11(5):803. doi: 10.3390/cells11050803
- Yu J, Fang T, Yun C, et al. Antibody-drug conjugates targeting the human epidermal growth factor receptor family in cancers. Frontiers in Molecular Biosciences. 2022;9:184. doi: 10.3389/fmolb.2022.847835
About Sino Biological Inc.

Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offers pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.