Antibody humanization involves modifying antibodies from non-human sources, such as mice, to decrease their immunogenicity in human applications.
While mouse monoclonal antibodies are specific, they are extremely immunogenic and can potentially induce human anti-mouse antibody (HAMA) responses, effectively compromising therapeutic effectiveness by reducing the antibodies’ half-life.
Substituting large segments of the mouse antibody molecule with human counterparts means that humanized antibodies have less chance of being recognized as foreign to the body, reducing the risk of HAMA reactions and improving treatment outcomes and patient safety.1
Evolution of therapeutic monoclonal antibodies
Therapeutic antibody development has evolved from mouse monoclonal antibodies to fully humanized forms, marking a major advancement in biotechnology.
Antibodies were initially derived directly from mice, and these antibodies exhibited significant immunogenicity in humans. The first generation of humanized antibodies - referred to as chimeric antibodies - saw the constant regions of a mouse antibody replaced with those of a human antibody, considerably reducing immunogenicity while maintaining the high specificity of the mouse antibody.
Subsequent generations, such as CDR-grafted antibodies, see the complementarity-determining regions (CDRs) of a mouse antibody transplanted onto a human framework, further lowering the risk of immunogenicity while better maintaining binding affinity.
Surface-reshaped antibodies are engineered by modifying the surface amino acids of mouse antibodies to minimize epitope recognition by the human immune system. This approach reduces immunogenicity without hindering the antigen-binding site.
Fully human antibodies can be generated via phage display technology or from human B cells. These antibodies are wholly derived from human sequences, resulting in the lowest immunogenicity risk while being ideally suited to repeated or long-term treatments.2
Development requirements for humanized antibodies
Humanized antibodies must meet strict development criteria to ensure safety and efficacy. These include high binding affinity, minimal immunogenicity, and considerable in vivo stability.
It is also important that these antibodies can be produced in large quantities under current Good Manufacturing Practices (cGMP). This approach ensures consistent quality and therapeutic effectiveness.3 Extensive biocompatibility testing is also necessary to confirm that antibodies do not elicit unintentional biological responses.
Antibody humanization strategies
Antibody humanization involves various complex techniques, each designed to reduce non-human antibodies’ immunogenicity while maintaining or improving their therapeutic efficacy. These methods are key to developing safe and effective antibodies for use in human medicine.
Complementarity-determining region (CDR) grafting
CDR grafting is the most commonly employed humanization method. This popular technique involves transferring CDRs from a non-human antibody to a human antibody framework.
CDRs are essential in ensuring antigen binding, so maintaining their integrity is important to maximize the antibody’s affinity and specificity.
The most notable challenge associated with CDR grafting is incorporating these regions into a human framework without inadvertently altering their structure. Several techniques are routinely used to ensure and validate the structural compatibility of the humanized CDRs within the human framework, including advanced molecular modeling and X-ray crystallography.4
Specificity-determining region (SDR) grafting
SDR grafting refines the focus on amino acids that directly interact with the antigen. These are the most critical parts within the CDRs, so modifying only these key areas ensures that SDR grafting minimizes any disruption to overall antibody structure and function, maintaining the antibody’s original binding properties more effectively than is possible with broader CDR grafting approaches.
Resurfacing approach
The resurfacing method modifies the antibody’s external amino acids to lower its immunogenicity without impacting the antigen-binding sites.
This approach works by replacing surface amino acids with variants more commonly found in humans, reducing the chance of recognition by the human immune system.
This approach is particularly useful in cases where preserving CDRs’ integrity is necessary to maintain binding affinity. Resurfacing can also be integrated with other grafting techniques to further optimize outcomes.
![Evolution of Humanization Methods: Starting with chimerization, the goal has been to minimize non-human elements without compromising functionality. This includes CDR grafting, where non-human CDR loops are grafted onto a human framework, SDR grafting, which involves grafting only the binding residues of the CDRs onto the framework, and resurfacing, where exposed non-human residues are replaced with human ones [1]](https://www.news-medical.net/images/appnotes/ImageForAppNote_5455_1732953369866183.jpg)
Figure 1. Evolution of Humanization Methods: Starting with chimerization, the goal has been to minimize non-human elements without compromising functionality. This includes CDR grafting, where non-human CDR loops are grafted onto a human framework, SDR grafting, which involves grafting only the binding residues of the CDRs onto the framework, and resurfacing, where exposed non-human residues are replaced with human ones 1. Image Credit: Sino Biological Inc.
Other antibody humanization methods
Antibody library technologies leverage comprehensive collections of human antibodies to locate sequences that can serve as effective scaffolds for humanization.
These libraries are typically generated either from B cells of immunized humans or through synthetic means like phage display technology. Meticulous screening is used to select antibodies that exhibit desirable traits, including low immunogenicity, high affinity, and specific effector functions.
This extremely versatile method enables the rapid evaluation and optimization of a large number of variants.
Another innovative approach involves using transgenic mice engineered to produce human antibodies upon exposure to antigens. These mice feature human immunoglobulin genes, allowing them to produce antibodies that are inherently human and, therefore, require minimal, if any, further humanization.
This approach provides a robust platform for the generation of fully human antibodies for a range of therapeutic applications.
Nanobodies are VHH segments derived from camelid antibodies. These single-domain antibodies are considerably smaller than traditional antibodies, and this compact size, coupled with their unique structure, enables access to cryptic epitopes that are inaccessible to conventional antibodies.
It is possible to substantially enhance these antibodies’ therapeutic potential via humanization, effectively reducing immunogenicity, improving tissue penetration, and making them especially beneficial to diagnostic applications and targeted cancer therapies.5
Applications of humanized antibodies
Humanized antibodies have been instrumental in transforming the treatment landscape for a range of diseases, particularly in terms of autoimmune disorders and oncology.
Because these antibodies are engineered to target antigens like tumors or pathological immune cells with high specificity, their use is key to minimizing side effects linked to traditional treatments. Humanized antibodies also play a critical role in vital diagnostic processes, where their specificity facilitates the detection of trace amounts of biomarkers.6
Sino Biological offers alpaca nanobody and murine monoclonal antibody humanization services that make use of complementarity-determining region (CDR) grafting technology and computer-aided molecular modeling. The company’s robust approach boasts a success rate of 100% and >95% sequence homology versus human antibody frameworks.
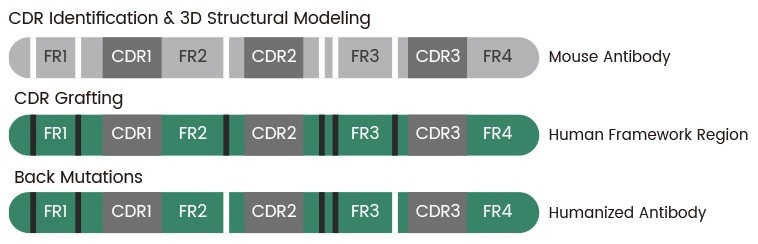
Figure 2. Complementarity-determining region (CDR) grafting technology as developed by Sino Biological. Image Credit: Sino Biological Inc.
Future prospects
The future of antibody humanization shows great promise. This innovative field is driven by ongoing advances in molecular biology, genetic engineering, and immunology.
It is anticipated that emerging techniques such as next-generation sequencing will further refine the efficacy and precision of humanized antibodies, and the ongoing development of universal platforms for rapid antibody humanization will reduce the costs and time associated with antibody development. These developments will ultimately enable more rapid responses to emerging health challenges.1
References and further reading
- Gordon, G.L., Matthew, Wong, A. and Deane, C.M. (2024). Prospects for the computational humanization of antibodies and nanobodies. Frontiers in Immunology, 15. https://doi.org/10.3389/fimmu.2024.1399438.
- Rossotti, M.A., et al. (2021). Immunogenicity and humanization of single‐domain antibodies. The FEBS Journal, 289(14), pp.4304–4327. https://doi.org/10.1111/febs.15809.
- Lu, R.-M., et al. (2020). Development of Therapeutic Antibodies for the Treatment of Diseases. Journal of Biomedical Science, 27(1), pp.1–30. https://doi.org/10.1186/s12929-019-0592-z.
- Safdari, Y., et al. (2013). Antibody humanization methods – a review and update. Biotechnology and Genetic Engineering Reviews, 29(2), pp.175–186. https://doi.org/10.1080/02648725.2013.801235.
- Ahmadzadeh, V., et al. (2014). Antibody Humanization Methods for Development of Therapeutic Applications. Monoclonal Antibodies in Immunodiagnosis and Immunotherapy, 33(2), pp.67–73. https://doi.org/10.1089/mab.2013.0080.
- Waldmann, H. (2018). Human Monoclonal Antibodies: The Benefits of Humanization. Methods in Molecular Biology, pp.1–10. https://doi.org/10.1007/978-1-4939-8958-4_1.
Acknowledgments
Produced from materials originally authored by Sino Biological.
About Sino Biological Inc.
Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.