Cytokines are key to immune regulation. Their extensive impact on human biology and disease has led to their long-term therapeutic use in treating cancer and a range of other diseases.
Interleukin-2 (IL-2), interferon-alpha (IFN-α), and other first-generation cytokines have historically been limited by high toxicity, short half-life, and lack of specificity. However, new developments in next-generation cytokines are ongoing, with the aim of improving stability, their potential for tumor targeting, and reducing toxicity.
The new developmental strategies include pegylation, immunocytokines, cytokine prodrugs, fusion proteins, and mRNA-based therapies.
Notable developments in recent years include ALT-803, NKTR-214, and L19-IL2, all showing particular promise in clinical trials. Despite these advances, challenges such as safety, manufacturing, combination therapies, and patient selection remain, and it is vital that these are addressed to ensure ongoing progress.
Cytokines and their role in immunotherapy
Cytokines are minute proteins with a central role in regulating immune cells’ growth, differentiation, and activation, meaning that they are vital for proper immune system functioning.1,2
Cytokines can also hinder tumor growth by stimulating immune cells to attack cells via direct antiproliferative effects. This ability affords cytokines significant potential in cancer immunotherapy.3,4
However, the use of natural cytokines in therapy has been historically limited due to their systemic toxicity, short half-life, and lack of specificity.5,6
First-generation cytokines
First-generation cytokines, such as IL-2 and IFN-α, represent a fundamental component of cancer immunotherapy. For example, IL-2 has been approved for the treatment of advanced renal cell carcinoma and metastatic melanoma, and IFN-α has been approved for follicular non-Hodgkin lymphoma, hairy cell leukemia, melanoma, and AIDS-related Kaposi’s sarcoma.3
All of these cytokines can bolster the immune system's T-cell and natural killer (NK) functions, making them ideally suited to helping combat tumors.5 Their widespread clinical use continues to be limited due to their severe toxicity at therapeutic doses, short in vivo half-life, and limited efficacy in the majority of patients.2,3
IL-2 can enhance T-cell proliferation and anti-tumor activity, but its use is accompanied by frequent and severe adverse effects in grades 3 and 4 before reaching therapeutic levels.3,5,6
Next-generation cytokines
Next-generation cytokines have been specifically engineered to enhance their predecessors’ properties, with innovations such as improved stability, lower toxicity, and enhanced specificity for tumor cells.
There are several key strategies employed in next-generation cytokine development.
Pegylation
Pegylation refers to the covalent bonding between polyethylene glycol (PEG) polymers and cytokines - a process that extends the stability and half-life of cytokines in the bloodstream.1
Pegylated cytokines can maintain therapeutic concentrations for extended periods, reducing administration frequency and limiting the risk of associated toxicities.2,7 Pegylation also expands cytokines’ hydrodynamic diameter, reducing renal clearance and immunogenicity.2
Fusion proteins
Active molecules' pharmacokinetics (PK) can be modified by covalently linking Fc fusion proteins to an immunoglobulin Fc domain. Fusing cytokines with antibodies or other targeting moieties allows researchers to specifically direct cytokine activity to tumor cells.
The Fc domain increases the fusion protein’s plasma half-life, enhancing its therapeutic efficacy and reducing the rate of renal macromolecular excretion.1 The Fc domain also binds to the Fcg receptor (FcgR) and complement, potentially contributing to antibody-dependent cell phagocytosis (ADCP), antibody-dependent cytotoxicity (ADCC), and complement-dependent cytotoxicity (CDC).8
Immunocytokines
Immunocytokines are antibody-cytokine fusion proteins with an amino acid linker, a targeting-antibody moiety, and a cytokine load. Immunocytokines may comprise cytokines fused to full-size antibodies or antibody fragments. These may also allow the molecule to target tumor-associated antigens.1,8,9
Cytokines activate and direct immune cells to tumor cells, promoting immune synapse formation. Activated immune cells reduce tumor cell proliferation and increase cytotoxicity.4 Immunocytokines have successfully enabled the localization of effector molecules in the TEM, expanding therapeutic strategies in this area.8
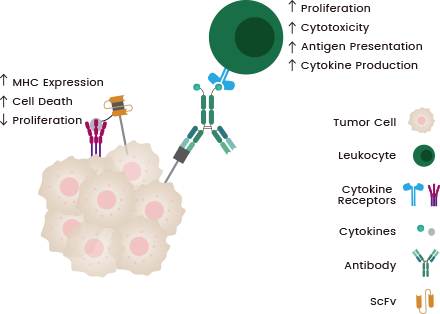
Figure 1. Mechanisms of anti-cancer action of immunocytokines.9 Image Credit: Sino Biological Inc.
Cytokine prodrugs
Prodrug cytokines comprise cytokine, masking moiety, specific ‘half-life extension elements,’ and an appropriate linker. Prodrug constructs represent an effective means of overcoming off-target effects.
In this approach, a peptide is linked to the cytokine, rendering it inactive. Proteases overexpressed at the tumor site can cleave this inactivating unit, prompting the cytokine's reactivation. Cytokine prodrug designs are typically paired with re-engineered cytokines, such as ProIL2, an inactive form of IL-2 6.
mRNA-based cytokine therapies
mRNA-based therapies involve the encoding and delivery of cytokines via synthetic mRNA to modulate the immune system. This developmental strategy enables the circumvention of toxicities linked to recombinant cytokine therapies.2
Lipid nanoparticles (LNPs) efficiently deliver mRNA to host cells, enabling cytokine production, with these cytokines then able to improve anti-tumor immunity, inhibit tumor growth, and modify the tumor microenvironment.10,11
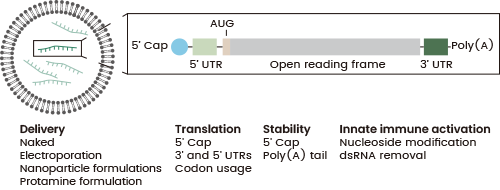
Figure 2. Delivery and Structural Elements of mRNA Therapeutics. Left: Lipid-based mRNA nanoparticle structure. Right: Synthetic mRNA structure with 5′ cap, 5′ and 3′ UTRs, start codon (AUG), and poly(A) tail. UTR: Untranslated region; ds: Double-stranded 11. Image Credit: Sino Biological Inc.
Examples of next-generation cytokines in development
A number of next-generation cytokines are currently under investigation via preclinical and clinical studies. These cytokines are showing promising results in terms of their ability to improve cancer immunotherapy.
ALT-803 (Nogapendekin alfa)
This IL-15 superagonist boasts improved stability and bioactivity compared to native IL-15. ALT-803 promotes the proliferation and activation of NK cells and memory CD8+ T cells, offering sustained anti-tumor activity.
It is also being evaluated in conjunction with a range of other immunotherapies, recently receiving FDA approval for the treatment of non-muscle invasive bladder cancer (NMIBC).12
L19-IL2 (Darleukin)
This diabody-format immunocytokine comprises two ScFv fragments and a C-terminal-fused IL-2. L19-IL2 enhances safety and therapeutic efficacy and can be co-treated with other therapeutics.
It is possible to completely eradicate β-cell lymphoma xenografts by using L19-IL2 as a single agent or in combination with L19-TNF immune cytokines or rituximab and CTLA-4 blockers.4
NKTR-214 (Bempegaldesleukin)
NKTR-214 is an engineered version of IL-2 designed to preferentially activate natural killer (NK) cells and CD8+ T cells, key components of the anti-tumor immune response.
NKTR-214 has demonstrated reduced binding to IL-2 receptors on regulatory T-cells (Tregs), which have the potential to suppress immune responses. Clinical trials have highlighted its potential for treating various cancers when used in conjunction with checkpoint inhibitors.13
Challenges and future directions
Cytokines are powerful immune mediators that represent a particular challenge for drug developers. Next-generation cytokines aim to address many of their most common issues via enhanced therapeutic efficacy with lower toxicity and longer half-lives.
The development and widespread implementation of next-generation cytokines face several challenges despite these promising advances.
Safety and toxicity
Cytokine therapies may still induce systemic toxicity, even with improved designs. It is vital that the safety of these treatments is ensured while maintaining their efficacy.4
Manufacturing and scalability
Next-generation cytokines rely on complex engineering and production processes, posing various manufacturing challenges. Scalability and cost-effectiveness represent key considerations in enabling the widespread clinical adoption of next-generation cytokines.2
Combination therapies
Careful optimization is required to successfully integrate next-generation cytokines with other cancer therapies, such as targeted therapies, checkpoint inhibitors, and adoptive cell transfer. It is essential that synergistic effects and potential interactions be fully understood if therapeutic outcomes are to be maximized.14
Sino Biological’s contribution to next-generation cytokine research
Sino Biological was named ‘Growth Factor Supplier to Watch in 2024’ by CiteAb. The company offers a comprehensive portfolio of recombinant cytokines for cell culture, improving and enabling research in tumor immunotherapy, drug screening, stem cell therapy, and regenerative medicine.
Its entire product range is subject to stringent quality control and comprehensive validation to ensure high purity, stability, biological activity, and low endotoxin levels.
Cytokines are available from several species, including rats, mice, and humans, all of which have been designed to support research into neural cells, stem cells, immune cells, and organoids.
Sino Biological is committed to delivering high-quality reagents for clinical research and drug development. To this end, the company offers research-use-only (RUO) and GMP-grade cytokines.
Developed under a GMP quality management system, its GMP-grade cytokines also offer improved stability and quality, supporting cell therapy and drug development processes throughout the field.

Image Credit: Sino Biological Inc.
References and further reading
- Holder, P.G., et al. (2022). Engineering interferons and interleukins for cancer immunotherapy. Advanced Drug Delivery Reviews, [online] 182, p.114112. https://doi.org/10.1016/j.addr.2022.114112.
- Deckers, J., et al. (2023). Engineering cytokine therapeutics. Nature Reviews Bioengineering, 1(4), pp.286–303. https://doi.org/10.1038/s44222-023-00030-y.
- Berraondo, P., et al. (2019). Cytokines in clinical cancer immunotherapy. British Journal of Cancer, [online] 120(1), pp.6–15. https://doi.org/10.1038/s41416-018-0328-y.
- Rybchenko, V.S., et al. (2023). Targeted Cytokine Delivery for Cancer Treatment: Engineering and Biological Effects. Pharmaceutics, [online] 15(2), p.336. https://doi.org/10.3390/pharmaceutics15020336.
- Xue, D. et al. (2021). Next-generation cytokines for cancer immunotherapy. Antibody Therapeutics, 4(2), pp. 123–133. https://doi.org/10.1093/abt/tbab014.
- Hsu, E.J. et al. (2021). A cytokine receptor-masked IL2 prodrug selectively activates tumor-infiltrating lymphocytes for potent antitumor therapy. Nature Communications, 12(1). https://doi.org/10.1038/s41467-021-22980-w.
- Dholakia, J., et al. (2022). Development of Delivery Systems for Local Administration of Cytokines/Cytokine Gene-Directed Therapeutics: Modern Oncologic Implications. Current Oncology Reports, 24(4), pp.389–397. https://doi.org/10.1007/s11912-022-01221-3.
- Fu, Y., Tang, R. and Zhao, X. (2023). Engineering cytokines for cancer immunotherapy: a systematic review. Frontiers in Immunology, 14. https://doi.org/10.3389/fimmu.2023.1218082.
- Runbeck, E., et al. (2021). Utilizing Immunocytokines for Cancer Therapy. Antibodies, 10(1), p.10. https://doi.org/10.3390/antib10010010.
- Pohl-Guimarães, F., et al. (2019). RNA-Modified T Cells Mediate Effective Delivery of Immunomodulatory Cytokines to Brain Tumors. Molecular Therapy, 27(4), pp.837–849. https://doi.org/10.1016/j.ymthe.2018.10.007.
- Beck, J.D., et al. (2021). mRNA therapeutics in cancer immunotherapy. Molecular Cancer, 20(1). https://doi.org/10.1186/s12943-021-01348-0.
- Chu, Y., et al. (2024). Efficiently targeting neuroblastoma (NB) by the combination of anti-ROR1 CAR NK cells and N-803 in-vitro and in-vivo of NB xenografts. Deleted Journal, 32(2), pp.200820–200820. https://doi.org/10.1016/j.omton.2024.200820.
- Kong, J.C. et al. (2024). Chimeric antigen receptor-natural killer cell therapy: current advancements and strategies to overcome challenges. Frontiers in Immunology, 15. https://doi.org/10.3389/fimmu.2024.1384039.
- Pan, C., et al. (2020). Next-generation immuno-oncology agents: current momentum shifts in cancer immunotherapy. Journal of Hematology & Oncology, 13(1). https://doi.org/10.1186/s13045-020-00862-w.
Acknowledgments
Produced from materials originally authored by Sino Biological.
About Sino Biological Inc.
Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.