CAR-NK cell therapies are innovative therapeutic options developed for the purpose of overcoming some of the limitations associated with CAR-T cell therapies, such as therapy-induced side effects.
Image Credit: Sino Biological Inc.
CAR-NK cells display higher cytolytic activity than memory T cells and untransduced NK cells, making them more effective at killing tumor cells. Today, more than 800 CAR-T and 28 CAR-NK cell clinical trials are being carried out globally.
Sino Biological is a global leading supplier of bioreagents and CRO services for the biopharmaceutical field. It offers a range of complete solutions for CAR-NK cell therapy development.
Sino’s reagents and services support its customers through all stages of the development process, from early target discovery to the preclinical phase of research and development.
What is CAR-NK cell therapy?
Natural killer (NK) cells are immune effector cells with innate potent cytotoxicity; they can eradicate abnormal cells, including tumors and virally infected cells.
CAR-NK cell therapy is a groundbreaking type of cancer immunotherapy for solid tumors and hematological malignancies, which leverages the use of NK cells.
Compared to CAR-T therapy, CAR-NK cell therapy is considered a safer alternative with a reduced chance of cytokine release syndrome and graft-versus-host disease while offering more efficient antitumor activity and greater efficiency for off-the-shelf production.
CAR-NK cells can be harvested from various sources, including peripheral blood (PB), umbilical cord blood (UCB), human-induced pluripotent stem cell, hematopoietic stem cells, human embryonic stem cells, and NK cell lines.
Sources and generation procedure of CAR-NK Cells:
- Isolated or established NK cells from various sources can be altered by CAR expression vectors (e.g., lentivirus).
- NK cells are subjected to expansion in a specific expansion media.
- The CAR-NK produced cells are typically injected intravenously to selectively kill tumor cells.
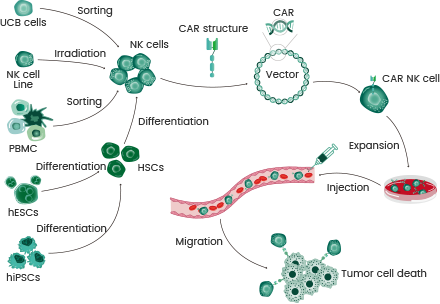
Reference: Faroogh Marofi1, et al. Cancer Science.2021.
Comprehensive solutions for CAR-NK cell therapy
Sino Biological offers an extensive range of solutions for the development of CAR-NK cells. The main factors in CAR-NK development include CAR development, NK cell collection and characterization, activation and expansion of NK cells, purification of NK cells, CAR-NK cell preparation, and quality control (QC).
Car development
The CARs are able to identify and bind to targeted antigens on the surface of tumor cells. Therefore, ensuring CARs of the highest quality is a crucial part of CAR development. Sino Biological cutting-edge technologies have enabled the development of four CAR development platforms for single-chain variable fragment (scFv) discovery.
To expedite the process of CAR molecule design in CAR-NK therapy research, Sino can offer choices from an extensive range of CAR-NK target proteins and SPR/BLI affinity characterization services for scFv screening and CAR affinity measurements.
CAR candidate development
CARs bind to target cells via a scFv which derives from antibodies, which is responsible for determining whether CAR-NK can recognize and kill specific tumor cells. Sino Biological offers various scFv development platforms, which cover the complete developmental process from antigen design and preparation to animal immunization to acquire the scFv molecules. These services allow Sino to meet the needs of its customers engaged in CAR-NK research.
Four scFv development platforms
Most scFv sequences of CAR-NK cells derive from mice and have specific immunogenicity. Therefore, scFv needs to undergo humanization processing.
Sino Biological offers top-class monoclonal antibody humanization services that can facilitate successful humanization with good results (>95%).
- 100% success rate
- Affinity validated by ELISA/SPR/BLI
- Rapid humanization: 3-4 weeks to deliver desired humanized antibodies
CAR candidate screening
scFv affinity characterization (SPR/BLI)
Among all CAR structures, a key element of CAR functionality is how scFv binds to its target antigen. Sino Biological offers SPR/BLI assay services which can screen appropriate scFv molecules effectively, thus supporting CAR-NK development with full effect.
- Authoritative reports support the IND or BLA filings of CAR-NK drugs
- Biacore and ForteBio Octet analysis platforms
- CNAS-accredited SPR platform
Assay cell line development
Sino Biological has the capacity to develop customized target antigen over-expression cell lines leveraging lentiviral vectors, supporting effective scFv screening by flow cytometry.
- 15+ years of experience in cell line development
- Complete analysis platform including FACS, qPCR, WB, and other identification methods
- Rapid delivery of stable cell lines in 2– 4 months
Target proteins
scFv screening is a vital component of CAR development. Sino Biological offers an extensive range of CAR-NK target proteins. These include popular solid tumor and malignant tumor proteins.
- 30+ hot CAR-NK target proteins, including HPLC-verified and site-specific biotinylated proteins
- FACS/ELISA/SPR-validated high activity
- High batch-to-batch consistency, which enables reproducibility
Antigen expression services
Target antigen selection is a key factor to determine CAR specificity and efficacy. Therefore, it is absolutely critical to ensure the appropriate target protein is chosen. Sino Biological offers tailor-made antigen design and preparation services to its customers, which fully supports their CAR-NK research requirements.
- Four expression systems: E. coli, mammalian, insect, and CHO/HEK293 stable cell line development
- HTP expression capabilities, supporting the rapid discovery of CAR-NK drugs
- Thorough QC testing: SDS-PAGE, SEC-HPLC, FACS, ELISA, cell assays, BLI/SPR, etc.
NK cell collection and characterization
NK cells can be acquired from a variety of sources, including PB, UCB, NK cell lines, and stem cell-derived NK cells. For that reason, NK cells must be isolated to eliminate other contaminants or components such as T or B cells.
NK cells are phenotypically characterized as lymphocytes expressing the antigens CD56 and CD16 (Fc gamma RIII), but missing CD3. Additionally, NK cells can be further sub-grouped into various populations based on the relative expression of the surface markers CD16 and CD56.
The two prime subsets are CD56brightCD16dim/− and CD56dimCD16+, respectively. To tell one apart from the other, antibodies targeting different NK cell surface proteins must be applied. Sino Biological offers a range of high-grade antibodies which facilitates NK cell isolation based on cell surface markers.
Featured antibodies for NK cell isolation
Anti-CD56 antibody (PE)
Cat#: 10673-MM05-P
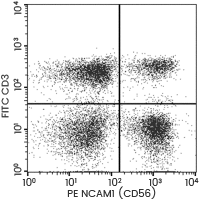
Flow cytometric analysis of Human NCAM1 (CD56) expression on human whole blood lymphocyte. Image Credit: Sino Biological Inc.
Anti-CD16 antibody (PE)
Cat#: 10389-MM41-P
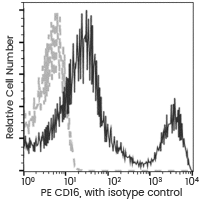
Flow cytometric analysis of Human CD16 expression on human whole blood lymphocyte. Image Credit: Sino Biological Inc.
More FACS antibodies for NK cell isolation
Source: Sino Biological Inc.
| Antigen |
Species |
Antibody type |
Cat# (Conjugate) |
Application |
| CD16 |
Human |
Mouse MAb
Rabbit MAb |
10389-MM22-C (PerCP)
10389-MM22-P (PE)
10389-MM22-F (FITC)
10389-MM22-A (APC)
10389-MM22 (Unconjugated)
10389-MM23-P (PE)
10389-MM23-A (APC)
10389-MM23-F (FITC)
10389-R221-F (FITC)
10389-MM41-F (FITC
11046-MM10-F (FITC) |
PBNK cell phenotyping |
| CD56 |
Human |
Mouse MAb
Rabbit MAb |
10673-R201-F (FITC)
10673-MM01-F (FITC)
10673-R201-P (PE)
10673-MM01-A (APC)
10673-MM05-F (FITC) |
| CD3 |
Human |
Mouse MAb
Rabbit MAb |
CT026-R301-A (APC)
CT026-R301-P (PE)
10977-M001-P (PE) |
| KIR2DL1 |
Human |
Rabbit MAb |
13145-R124-C (PerCP)
13145-R124-P (PE)
13145-R124-A (APC)
13145-R124-F (FITC)
13145-R124 (Unconjugated) |
NK cell line phenotyping |
| CD25 |
Human |
Mouse MAb
Rabbit MAb |
10165-MM17-C (PerCP)
10165-MM17-P (PE)
10165-MM17-F (FITC)
10165-MM17T (Unconjugated)
10165-R216-A (APC)
10165-R216-P (PE)
10165-R216-C (PerCP)
10165-R216-F (FITC)
10165-R216 (Unconjugated) |
UCB cell phenotyping |
NK cell activation and expansion
NK cells only make up a tiny fraction of PB leukocytes. Therefore, yielding acceptable numbers of NK cells poses a significant challenge for adoptive immunotherapy in humans.
Efficient NK cell transduction starts with physiological NK cell activation to provoke cell growth. Despite the various methods available today which promote NK cell proliferation, the two most commonly used approaches are feeder cells and cytokine mixtures.
GMP-grade cytokines
Cytokines are key components for the in vivo development and survival of NK cells; IL-21 supports the growth and boosts the cytotoxic function of NK cells. To develop CAR-NK drugs, GMP-grade cytokines are leveraged as raw materials to activate NK cells effectively throughout in vitro expansion.
Sino Biological is compliant when adhering to GMP guidelines for cytokine production and has developed high-purity and activity GMP-grade cytokines, such as IL-2, IL-7, IL-12, IL-15, and IL-21 with relative success rates.
Four different systems ensure high-quality, GMP-grade cytokine production to support CAR-NK product manufacturing
Quality management system
- Compliance with Good Manufacturing Practice
- Compliance with ISO 9001:2015 and ISO 13485:2016 system
- Full regulatory support files (RSF)
- FDA DMF filed
Quality control system
- Compliance with the General Chapter of Chinese Pharmacopoeia, 2020 Edition, Volume III
- Compliance with USP<1043>Ancillary Materials For Cell, Gene, and Tissue-engineered Products
- Compliance with USP Chapter <92> Growth Factors and Cytokines Used in Cell Therapy Manufacturing
Quality assurance management system
- Personnel and training
- Raw material and supplier
- Facility and equipment
- Document and record
- All documents and records are reviewed or approved by QA
Product safety testing system
- Bacterial endotoxins less than 5-10 EU/mg
- Residual host cell protein less than 0.5 μg/mg
- Residual host cell DNA less than 100 pg/mg
- Mycoplasma testing
- Sterility testing
- Adventitious virus testing
- Abnormal toxicity testing
Large-scale manufacturing capabilities
4,000 m2 of GMP cleanroom facilities

Image Credit: Sino Biological Inc.
Advanced culture and purification equipment

Image Credit: Sino Biological Inc.
GMP-grade cytokines for CAR-NK culture
Source: Sino Biological Inc.
| Target |
Cat# |
SEC-HPLC |
Endotoxin |
Mycoplasma Testing |
Sterility Testing |
Residual DNA |
Residual HCP |
| IL-2 |
GMP-11848-HNAE |
≥95% |
< 0.005
EU/μg |
Negative |
NO
Growth |
<100 pg/mg |
<0.5
μg/mg |
| IL-7 |
GMP-11821-HNAE |
≥95% |
< 0.01
EU/μg |
Negative |
NO
Growth |
<100 pg/mg |
<0.5
μg/mg |
| IL-12 |
GMP-CT011-H08H |
≥95% |
< 0.005
EU/μg |
Negative |
NO
Growth |
<100 pg/mg |
<0.5
μg/mg |
| IL-15 |
GMP-10360-HNAE |
≥95% |
< 0.01
EU/μg |
Negative |
NO
Growth |
<100 pg/mg |
<0.5
μg/mg |
| IL-21 |
GMP-10584-HNAE |
≥95% |
< 0.005
EU/μg |
Negative |
NO
Growth |
<100 pg/mg |
<0.5
μg/mg |
CAR-NK cell preparation
Lentiviral packaging plays a key role in the development of CAR-NK cell products. A range of different factors influence the success of lentiviral packaging. These include the vector system being used, as well as which transfection reagents and lentiviral titers are employed.
Sino Biological’s years of experience in virus packaging has led to the development of an HEK293 serum-free medium, Sinofection® transfection reagent, and SuperNuclease. These products ensure high virus titer and transfection efficiency. Sino also offers cell bank testing services and SuperNuclease ELISA Kits to meet the various needs of CAR-NK and cell therapy researchers.
Lentivirus packaging system
Lentiviral vector introduction efficiency has an impact on the quality of CAR-NK therapeutic products. Optimally, the vector should possess high transfection efficiency and high stability.
Sino Biological supplies a variety of transfection reagents, serum-free media, and supplements, all manufactured under strict QC guidelines.
GMP-grade SMM 293-CD1 medium (cat#: M293CD1)
- Chemically defined, serum-free, and animal component-free
- Appropriate for lentiviral packaging to support CAR-NK drug development
- Supportive SMS 293-SUPI supplement(Cat#: M293-SUPI)
Nucleic acid removal and detection
Certain safety issues and concerns around drug efficacy arise due to the residual presence of the DNA of host cells. This is an impurity associated with the production of CAR-NK cell drugs. Both the high-grade SuperNuclease and SuperNuclease ELISA Kit developed independently by Sino Biological can efficiently detect the amount of host DNA residue residing in CAN-NK cell samples.
GMP-grade superNuclease (cat#: GMP-SSNP01)
- FDA DMF filed (No. 35978), supporting your IND, NDA, and BLA
- High performance: HPLC>99%, enzyme activity >1.1×106 U/mg
- Improved specific activity than other leading manufacturers
- Strict quality control: endotoxin, sterility, mycoplasma, residual HCP, etc.
SuperNuclease ELISA kit (cat#: KIT-SSNP01)
- Broad applicability: detection of residual endonucleases from a range of suppliers
- Full methodological validation reports
- High sensitivity and the lowest detection limit is 14.55 pg/mL
- High batch-to-batch consistency
- High precision: CV <15%
Car-NK quality control
To guarantee the safety of CAR-NK cell products, robust quality control testing must be in place, including evaluation of cell population profiling, cell viability, CAR detection, and cytotoxicity analysis. Sino Biological offers an extensive range of products and services to facilitate the QC testing of CAR-NK cells.
Source: Sino Biological Inc.
| Assays |
Purpose |
Solutions |
| Cell population profiling |
Monitoring CAR-NK cell activation, differentiation, exhaustion, etc. |
A wide panel of FACS antibodies, used in:
- NK cell phenotype identification: CD3, CD56, and CD16
- NK cell activation markers: CD25, CD69, and CD107a
- NK cell exhaustion marker: PD-1
|
| CAR detection |
Understanding the key features of CAR-NK products |
- CAR-NK target proteins: HPLC and site-specific biotinylated, and other formats
- Protein L: universal detection and low-cost
- Anti-idiotype antibody services: specifically designed for scFv, with high specificity
|
| Detection of non-target cells |
Detecting percentage of residual tumor cells |
- High-quality antibodies against CAR-NK targets, including CD19, CD22, EGFR, and PD-L1.
|
| Detection of residual cytokines |
Detecting residual cytokines |
- Human IL-2 ELISA Kit (Cat#: KIT11848)
- Human IL-7 ELISA Kit (Cat#: KIT11821)
- Human IL-12 ELISA Kit (Cat#: KITCT011)
|
| Cytotoxicity evaluation |
Detecting CAR-NK cell cytotoxicity |
- ELISA Kits
- Assay cell line development service
|
Solutions to facilitate the development of CAR-NK cell therapy
Natural killer (NK) cells are immune effector cells with innate potent cytotoxicity; thus, they can eradicate abnormal cells, including tumor cells and virally infected cells. CAR-NK cell therapy is a groundbreaking type of cancer immunotherapy for solid tumors and hematological malignancies, which leverages the use of NK cells.
To ensure the safety and quality of CAR-NK cells, the entire manufacturing process of CAR-NK cells, including CAR development, NK cells collection, and CAR-NK cells preparation, demands stringent control measures and high-grade reagents. Sino Biological addresses any obstacles associated with the creation of CAR-NK cells and offers solutions to enhance the development process.
Natural Killer Cell Online Symposium
Video Credit: Sino Biological Inc.
About Sino Biological Inc.
Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.