Protein kinase inhibitors represent a major advance in cancer treatment due to their ability to target the specific kinases involved in tumor growth and survival. More than 80 small-molecule protein kinase inhibitors received FDA approval by 2024, with the majority of these inhibiting ATP binding to the target kinase.1,2
The development of resistance often limits inhibitors’ effectiveness; however, this resistance is primarily due to mutations in the kinase domain. Next-generation kinase inhibitors are being developed to help address this challenge, better tackling drug resistance and enhancing patient outcomes.2,3,4
Physiological and pathological importance of protein kinases
Protein kinases are essential enzymes able to regulate a diverse array of cellular processes by catalyzing protein phosphorylation. This involves transferring the γ-phosphate from ATP to specific amino acid residues of the protein substrates.5
Phosphorylation is one of the most critical and widely used post-translational modifications (PTMs) in eukaryotes due to its ability to alter the biological activity of proteins performing various cell roles.
Protein kinases function as molecular switches within sophisticated networks of signals, taking on an important regulatory role in almost every aspect of cell life, including cell growth, metabolism, division, apoptosis, signal transduction, and differentiation.3,5
From a physiological perspective, protein kinases maintain overall cellular homeostasis by ensuring proper metabolic balance, cell cycle progression, and cellular responses to external signals.5
Dysregulation of protein kinases can result in pathological conditions, however. For example, abnormal kinase activity is a hallmark of a number of cancers, with mutations in kinases like EGFR and BRAF driving uncontrolled cell proliferation and survival (Figure 1).3,6,7
Protein kinase dysfunction is also implicated in neurodegenerative disorders, cardiovascular diseases, and diabetes, further highlighting their key role in disease progression.8,9
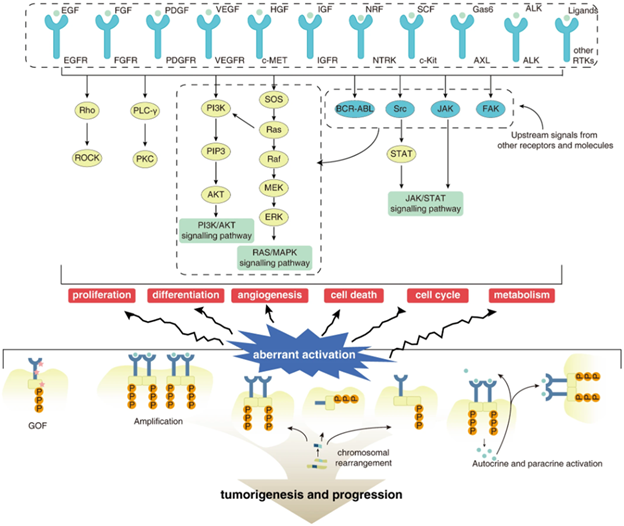
Figure 1. Abnormal Activation of Protein Tyrosine Kinases (PTKs) in Tumors. Figure source: https://doi.org/10.1038/s41392-022-01168-8
It is important to note that proper PTK activation and inactivation are key to normal cellular function, with aberrant PTK activation mediated by four major mechanisms: genomic amplifications, gene fusions, gain-of-function mutations, and autocrine/paracrine ligand loops.3
Kinase inhibitors approved for clinical use
Dysregulation of protein kinase activity plays a central role in the pathogenesis of cancer and other diseases. Because of this, protein kinases have become a leading target class for therapeutic intervention.3
Developing kinase inhibitors has proven revolutionary in cancer treatment, with drugs like erlotinib and imatinib demonstrating substantial clinical success.
These inhibitors block the kinase’s ATP-binding site, preventing the enzyme from phosphorylating its substrates and halting the signaling pathways that drive cancer cell growth and survival.3,10
Protein kinases’ versatility as drug targets extends beyond oncology to include inflammatory, cardiovascular, and neurodegenerative diseases.9
Table 1. FDA-Approved Small-Molecule Protein Kinase Inhibitors from 2018 to September 2024. Source: Sino Biological Inc.
Kinase
Classe |
Kinase
Group |
Primary
Targets/
Family |
2024
(2 Drugs) |
2023
(7 Drugs) |
2022
(4 Drugs) |
2021
(7 Drugs) |
2020
(8 Drugs) |
2019
(6 Drugs) |
2018
(9 Drugs) |
Receptor
Tyrosine
Kinases
(RTKs) |
TK |
EGFR/
ERBB |
Lazertinib |
|
|
Mobocertinib |
Tucatinib |
|
Dacomitinib |
| FGFR |
|
|
Futibatinib |
Infigratinib |
Pemigatinib |
Erdafitinib |
|
| VEGFR |
|
Fruquintinib |
|
Tivozanib |
|
|
|
| FLT3 |
|
Quizartinib |
|
|
|
|
Gilteritinib |
| RET |
|
|
|
|
Prasetinib
Selpercatinib |
|
|
KIT/
PDGFRA |
|
|
|
|
Avapritinib
Ripretinib |
|
|
| MET |
|
|
|
Tepotinib |
Capmatinib |
|
|
| TRK |
|
|
|
|
|
Entrectinib |
Larotrectinib |
| ALK |
|
|
|
|
|
|
Lorlatinib |
| CSFIR |
|
|
|
|
|
Pexidartinib |
|
| ROSI |
|
Repotrectinib |
|
|
|
|
|
Nonreceptor
Tyrosine
Kinases
(NTKS) |
JAK |
|
Momelotinib
Ritlecitinib |
Abrocitinib
Deucravacitinib
Pacritinib |
|
|
Fedratinib
Upadacitinib |
Baricitinib |
| BTK |
|
Pirtobrutinib |
|
|
|
Zanubrutinib |
|
| BCR-ABL |
|
|
|
Asciminib |
|
|
|
| SYK |
|
|
|
|
|
|
Fostamatinib |
Serine/
Threonine
Kinases
(STKS) |
AGC |
ROCKI/
ROCK2 |
|
|
|
Belumosudil |
|
|
Netarsudil |
| AKT |
|
Capivasertib |
|
|
|
|
|
| TKL |
BRAF |
Tovorafenib |
|
|
|
|
|
Encorafenib |
| CMGC |
CDK4/
CDK6 |
|
|
|
Trilaciclib |
|
|
|
Dual
Specificity
Kinases
(DSKS) |
STE |
MEKI/
MEK2 |
|
|
|
|
Selumetinib |
|
Binimetinib |
Please view detailed information on FDA-approved kinase inhibitors over the past two decades here. SignalChem Biotech - now part of Sino Biological - provides wild-type and mutant kinases for all primary targets of FDA-approved kinase drugs. This facilitates the development of next-generation kinase inhibitors with improved selectivity and potential to overcome drug resistance.
Acquired point mutations within the kinase domain that diminish inhibitor potency represent a prevalent mechanism of resistance to kinase inhibitors.
These mutations can potentially reduce the inhibitor’s inherent affinity for the target kinase by inducing a conformational change in the kinase or eliminating critical interactions.3,11 Resistance mutations may also increase the kinase’s affinity for ATP, lowering ATP-competitive inhibitors’ relative cellular potency.
Cancer cells may also activate alternative signaling pathways to bypass the inhibited kinase, an off-target resistance mechanism. This is known as ‘bypass signaling,’ allowing cancer cells to grow and survive even with the inhibitor present.12
An in-depth understanding of these mechanisms is key to developing next-generation inhibitors and combination therapies to overcome resistance and enhance treatment outcomes.3,13
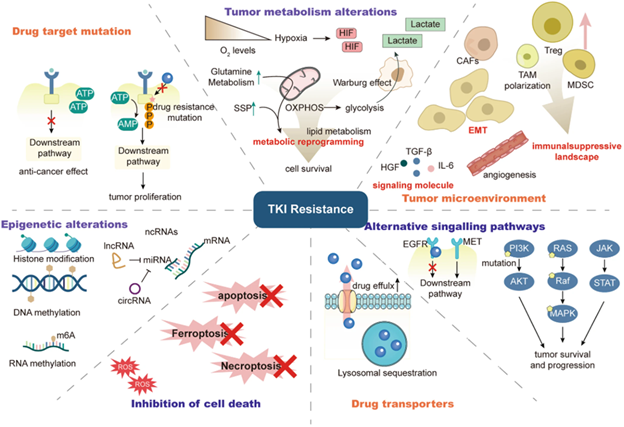
Figure 2. Mechanisms of Tumor Resistance to Tyrosine Kinase Inhibitors (TKIs). Figure source: https://doi.org/10.1038/s41392-022-01168-8
Various mechanisms can prompt tumor resistance to TKIs. Direct mechanisms include mutations in the targets that prevent drug binding, while tumors also exhibit enhanced drug efflux or lysosomal sequestration that reduces intracellular kinase inhibitor levels.
The tumor microenvironment (TME) also supports cancer growth, promoting adaptive tumor cell changes like metabolic reprogramming and epithelial-mesenchymal transition (EMT), or inducing immunosuppression.
Tumors may bypass protein kinase dependency or activate alternative signaling pathways. It is also important to note that epigenetic regulation further affects these resistance mechanisms.3
Next-generation kinase inhibitors
Next-generation kinase inhibitors’ effectiveness stems from their ability to overcome resistance mechanisms. Several strategies are used to do this.
Targeting mutant kinases
Targeting mutant kinases are compounds specifically designed to inhibit drug-resistant mutant forms of kinases. LOXO-195 is an example of a next-generation TRK inhibitor able to effectively inhibit clinically observed TRK resistance mutations, including TRKA-G667C, TRKA-G595R, and TRKC-G623R.14
Allosteric inhibitors
Unlike classical ATP-competitive inhibitors, allosteric inhibitors bind outside the target kinase’s catalytic domain/ATP-binding site, inhibiting its activity by inducing a conformational change. Allosteric inhibitors represent a promising example of next-generation therapeutics because they remain unaffected by common ATP-site resistance mutations.2,15
Covalent inhibitors
These inhibitors tend to form durable covalent bonds with kinase-reactive residues such as nucleophilic residues like cysteine or lysine, enhancing binding affinity and selectivity. Covalent inhibitors have the potential to be highly effective against kinases with mutations that confer resistance to prior-generation inhibitors.2,16
Combination therapies
A further promising means of overcoming and preventing resistance stems from the design of kinase inhibitor combinations and the combination of kinase inhibitors with other therapeutic modalities.
For example, achieving the dual inhibition of oncogenic signaling by targeting multiple nodes within a pathway, such as combining MEK and BRAF inhibitors to delay resistance in malignant melanoma, is possible.17
Table 2. Examples of Next-Generation Kinase Inhibitors Designed to Overcome Resistance. Source: Sino Biological Inc.
| Inhibitor |
Target |
Mechanism |
Clinical Indication(s) |
| LOXO-19514 |
TRK |
Targets mutant TRK kinases |
Overcomes resistance in TRK fusion-positive cancers |
| Osimertinib18 |
EGFR |
Covalent inhibitor |
Effective against EGFR T790M mutation in NSCLC |
| Avapritinib19 |
KIT,
PDGFRA |
Targets mutant kinases |
Treats GIST with PDGFRA D842V mutation |
| Asciminib20 |
BCR-ABLI |
Allosteric inhibitor |
Effective in CML resistant to ATP-competitive inhibitors |
| Entrectinib21 |
TRK, ROSI, ALK |
Multi-targeted inhibitor |
Treats cancers with TRK, ROSI, or ALK fusions |
Next-generation protein kinase inhibitors have the potential to revolutionize cancer therapy. Clinical trials have already shown extremely promising results, with next-generation inhibitors showing efficacy in patients exhibiting resistance to prior-generation drugs.6,18,19,20,21
These pioneering new drugs could potentially improve patient outcomes and provide more effective treatment options for a range of cancers, thanks to their ability to successfully address the limitations of early-generation kinase inhibitors and their capacity to overcome resistance mechanisms.
Kinase drug discovery solutions
SignalChem Biotech (part of Sino Biological) has developed one of the largest selections of mutant kinases available worldwide. The company leverages this selection to support ongoing research into disease-associated kinase mutations, identify mutant-selective inhibitors, and better understand the mechanisms of acquired resistance.
This range of clinically relevant mutant kinases has been produced with strict quality control to ensure high activity and excellent lot-to-lot consistency.
SignalChem Biotech is a world leader in kinase manufacturing and assays. The company remains closely engaged in every key aspect of kinase drug discovery, having supported researchers in academia and industry worldwide for over 20 years.
SignalChem Biotech offers the world's most comprehensive and wide-ranging portfolio of highly active kinases (700+ wild-type and 400+ mutant kinases), swift compound screening and profiling services, and custom enzyme and assay development.
The high activity of these kinases ensures dependable screening and profiling results and extensive coverage of more than 90% of the human kinome.
References and further reading
- Min, H.-Y. and Lee, H.-Y. (2022). Molecular targeted therapy for anticancer treatment. Experimental & Molecular Medicine, [online] 54(10), pp.1670–1694. https://doi.org/10.1038/s12276-022-00864-3.
- Li, J., et al. (2024). Kinase Inhibitors and Kinase-Targeted Cancer Therapies: Recent Advances and Future Perspectives. International Journal of Molecular Sciences, [online] 25(10), p.5489. https://doi.org/10.3390/ijms25105489.
- Yang, Y., et al. (2022). Protein tyrosine kinase inhibitor resistance in malignant tumors: molecular mechanisms and future perspective. Signal Transduction and Targeted Therapy, [online] 7(1). https://doi.org/10.1038/s41392-022-01168-8.
- Braun, T.P., Eide, C.A. and Druker, B.J. (2020). Response and Resistance to BCR-ABL1-Targeted Therapies. Cancer Cell, 37(4), pp.530–542. https://doi.org/10.1016/j.ccell.2020.03.006.
- Cheng, H.-C., et al. (2011). Regulation and Function of Protein Kinases and Phosphatases. Enzyme Research, [online] 2011. https://doi.org/10.4061/2011/794089.
- Bhullar, K.S., et al. 2018). Kinase-targeted cancer therapies: progress, challenges and future directions. Molecular Cancer, 17(1). https://doi.org/10.1186/s12943-018-0804-2.
- Grant, S.K. (2008). Therapeutic Protein Kinase Inhibitors. Cellular and Molecular Life Sciences, 66(7), pp.1163–1177. https://doi.org/10.1007/s00018-008-8539-7.
- Geraldes, P. and King, G.L. (2010). Activation of Protein Kinase C Isoforms and Its Impact on Diabetic Complications. Circulation Research, [online] 106(8), pp.1319–1331. https://doi.org/10.1161/circresaha.110.217117.
- Shmuel Silnitsky, et al. (2023). An Update on Protein Kinases as Therapeutic Targets—Part I: Protein Kinase C Activation and Its Role in Cancer and Cardiovascular Diseases. International Journal of Molecular Sciences, [online] 24(24), pp.17600–17600. https://doi.org/10.3390/ijms242417600.
- Pottier, C., et al. (2020). Tyrosine Kinase Inhibitors in Cancer: Breakthrough and Challenges of Targeted Therapy. Cancers, [online] 12(3), p.731. https://doi.org/10.3390/cancers12030731.
- Barouch-Bentov, R. and Sauer, K. (2011). Mechanisms of drug resistance in kinases. Expert Opinion on Investigational Drugs, 20(2), pp.153–208. https://doi.org/10.1517/13543784.2011.546344.
- Lin, J.J. and Shaw, A.T. (2016). Resisting Resistance: Targeted Therapies in Lung Cancer. Trends in Cancer, [online] 2(7), pp.350–364. https://doi.org/10.1016/j.trecan.2016.05.010.
- De Santis, S., et al. (2022). Overcoming Resistance to Kinase Inhibitors: The Paradigm of Chronic Myeloid Leukemia. OncoTargets and Therapy, Volume 15, pp.103–116. https://doi.org/10.2147/ott.s289306.
- Drilon, A. et al. (2017). A Next-Generation TRK Kinase Inhibitor Overcomes Acquired Resistance to Prior TRK Kinase Inhibition in Patients with TRK Fusion–Positive Solid Tumors. Cancer Discovery, 7(9), pp. 963–972. https://doi.org/10.1158/2159-8290.cd-17-0507.
- Schoepfer, J. et al. (2018). Discovery of asciminib (ABL001), an allosteric inhibitor of the tyrosine kinase activity of BCR-ABL1. Journal of Medicinal Chemistry, 61(18), pp. 8120–8135. https://doi.org/10.1021/acs.jmedchem.8b01040.
- Ou, X., et al. (2024). Mechanisms of resistance to tyrosine kinase inhibitor-targeted therapy and overcoming strategies. MedComm, [online] 5(9), p.e694. https://doi.org/10.1002/mco2.694.
- Cohen, P., Cross, D. and Jänne, P.A. (2021). Kinase Drug Discovery 20 Years after imatinib: Progress and Future Directions. Nature Reviews Drug Discovery, 20(7). https://doi.org/10.1038/s41573-021-00195-4.
- Leonetti, A., et al. (2019). Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. British Journal of Cancer, [online] 121(9), pp.725–737. https://doi.org/10.1038/s41416-019-0573-8.
- Teuber, A. et al. (2024). Avapritinib-based SAR studies unveil a binding pocket in KIT and PDGFRA. Nature Communications, 15(1). https://doi.org/10.1038/s41467-023-44376-8.
- Réa, D., and Hughes, T. P. (2022). Development of asciminib, a novel allosteric inhibitor of BCR-ABL1. Critical Reviews in Oncology/Hematology, 171, 103580. https://doi.org/10.1016/j.critrevonc.2022.103580
- Jiang, Q., et al. (2022). Entrectinib, a new multi-target inhibitor for cancer therapy. Biomedicine & Pharmacotherapy, [online] 150, p.112974. https://doi.org/10.1016/j.biopha.2022.112974.
Acknowledgments
Produced from materials originally authored by Sino Biological.
About Sino Biological Inc.
Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.