Cytokines are secreted proteins critical for immune regulation, cell signaling, and tissue homeostasis. These cells are increasingly recognized as key tools in biomedical research, particularly in drug discovery and regenerative medicine.
Cytokines play a key role in cellular processes, including proliferation, differentiation, and apoptosis, making them crucial for researching disease mechanisms and therapeutic use cases.1,2
The emergence of sophisticated in vitro models, like induced pluripotent stem cells (iPSCs) and organoids, has greatly improved the utility of cytokines in imitating complex tissue microenvironments.3 However, cytokines’ pleiotropic nature—where a single cytokine impacts several biological pathways—poses challenges and thus necessitates fine-tuned modulation to fully exploit their therapeutic potential.4
Cytokines in stem cell biology
Cytokines are crucial for sustaining stem cell pluripotency and self-renewal and for driving their differentiation into specific lineages. iPSCs, derived from somatic cells through reprogramming, depend on a carefully controlled microenvironment with cytokines, including FGF2, TGF-β, and BMP4 to maintain their pluripotent state.5,6,7
During differentiation, cytokine signaling enables lineage specificity by imitating embryonic development pathways. For example, Activin A and BMP2 underlie the development of endodermal tissues, including the liver and pancreas, while VEGF and SCF guide mesodermal differentiation into hematopoietic and vascular cells.6,7
The strategic use of cytokines additionally addresses the variability in differentiation protocols, enabling consistent generation of desired cell types. This has made iPSCs and organoids derived from them valuable tools for disease modeling, personalized medicine, and regenerative therapies.8
Alongside differentiation, cytokines are key for sustaining both stem cells' genomic stability and functional integrity during expansion. Their ability to influence critical pathways such as WNT and Notch highlights their importance in stem cell research.9
Organoids as a cytokine-driven research platform
Organoids—three-dimensional cell culture systems that imitate human organs’ structure and function—have arisen as transformative tools in drug discovery. Originating from stem cells or tissue biopsies, organoids depend on cytokines to generate organ-specific microenvironments (Fig. 1).
In intestinal organoids, Wnt3a and EGF facilitate epithelial proliferation, while BMP4 regulates maturation.10 Similarly, VEGF and FGF2 are critical for vascularized brain organoids, enabling the development of functional blood vessels.11
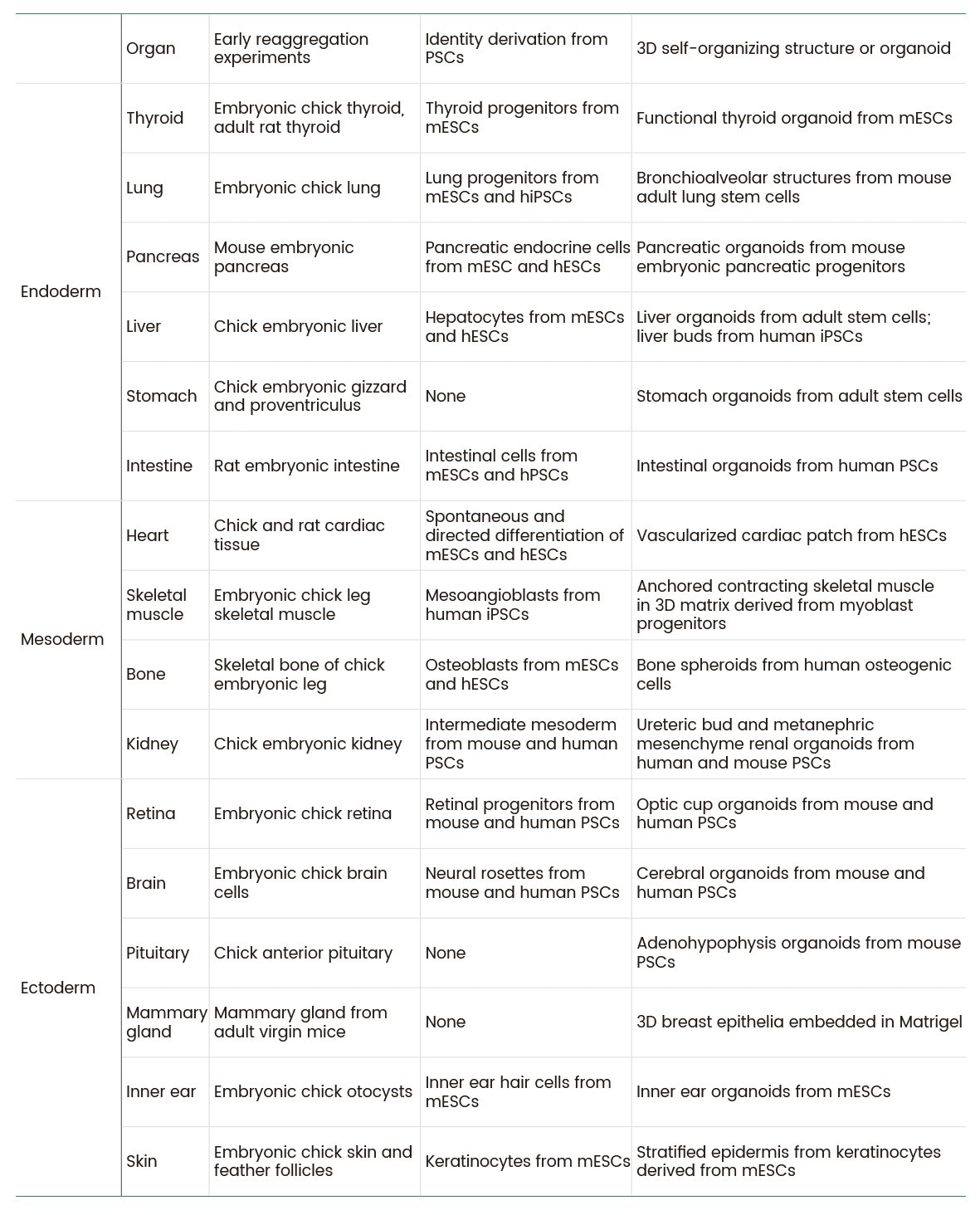
These cytokine-driven models deliver unprecedented insights into developmental processes, disease mechanisms, and host-pathogen interactions, providing a physiologically relevant alternative to conventional 2D cultures (Table 1).
Integrating organoids with cytokine signaling has facilitated researchers in simulating complex conditions, including inflammation, fibrosis, and tumor progression. By using cytokines, including IL-6 and TNF-α, organoids can recreate inflammatory microenvironments, which are critical for studying autoimmune conditions and chronic inflammatory diseases.12,13
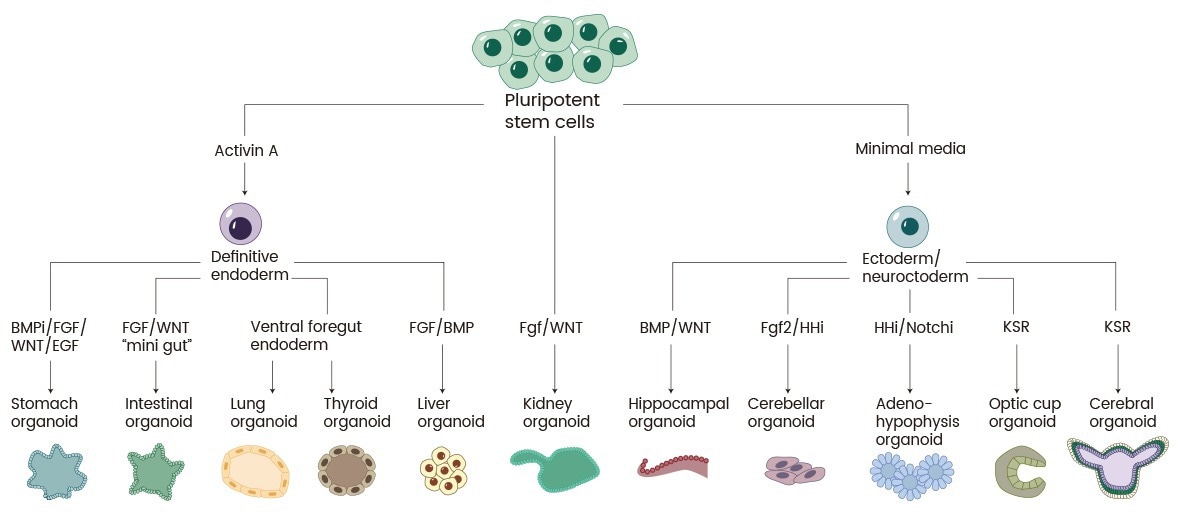
Figure 1. Schematic of the various organoids that can be grown from PSCs and the developmental signals that are employed1. Image Credit: Sino Biological Inc.
Table 1. In vitro self-organizing tissues of various organs8. Source: Sino Biological Inc.
Expanding the role of cytokines in drug discovery
Integrating cytokines into stem cell and organoid research has greatly improved drug discovery by facilitating more precise, predictive, and personalized models. Cytokine-enhanced systems produce important platforms for drug screening, toxicity testing, and mechanistic research.
Personalized medicine and disease modeling
Patient-derived iPSCs and cytokine-supported organoids provide unparalleled opportunities for research in personalized medicine. Cytokines, including IL-6 and IL-1β, recreate inflammatory conditions to assess drug responses in patient-specific contexts. For instance, cystic fibrosis organoids that contain Wnt signaling modulators facilitate precise testing of therapeutic candidates customized to individual genetic profiles.14
Immunotherapy development
Cytokines are key for advancing immunotherapy research. Organoid models, including immune cells and cytokines, including IL-2 and IL-15, facilitate the recreation of tumor microenvironments. These systems enable the examination of immune checkpoint inhibitors, cytokine-based therapies, and adoptive T-cell strategies, offering crucial insights into the dynamic interactions between tumors and the immune system.15
High-throughput screening
Organoid platforms, together with cytokine-driven differentiation protocols, enable high-throughput drug screening. For example, liver organoids supported by VEGF and HGF are used in high-throughput hepatotoxicity screening, enabling the rapid assessment of drug safety profiles throughout preclinical evaluations.16
Likewise, lung organoids that contain FGF2 and EGF have a critical role in high-throughput respiratory disease modeling and antiviral therapy testing, facilitating efficient evaluation of many compounds.17
Infectious disease research
Cytokines are critical for the study of infectious diseases. During the COVID-19 pandemic, cytokine-driven lung and intestinal organoids delivered crucial insights into SARS-CoV-2 pathogenesis, such as the cytokine storm that leads to severe disease outcomes.18
Regenerative medicine
Cytokines additionally support the use of organoids in regenerative medicine. Pancreatic organoids, supported by cytokines including Activin A, FGF2, and IGF-1, are currently being investigated for their potential in treating diabetes.7 Hepatic organoids are also under development to model liver regeneration and test cell-based therapies.
Challenges and future directions
While use cases for cytokines in stem cell and organoid research are increasing, many challenges remain. Cytokine pleiotropic effects can result in undesirable off-target effects, which underscore the need for precision engineering of cytokine formulations (Fig. 2).4 Moreover, recombinant cytokines' elevated cost and batch variability remain hurdles to wider use in industrial use cases.
New solutions include the development of synthetic cytokine mimetics and engineered cytokines with improved specificity and stability. Advances in bioengineering, including microfluidics and 3D bioprinting, are additionally set to enhance the scalability and reproducibility of cytokine-driven systems. Moreover, using cytokine-based platforms alongside technologies such as single-cell sequencing and CRISPR genome editing will enable scientists to optimize therapeutic strategies.
Cytokine research continues to develop, producing exciting opportunities to apply basic science in clinical settings. By addressing current constraints, cytokines will maintain their place as critical tools in the ongoing transformation of drug discovery and regenerative medicine.
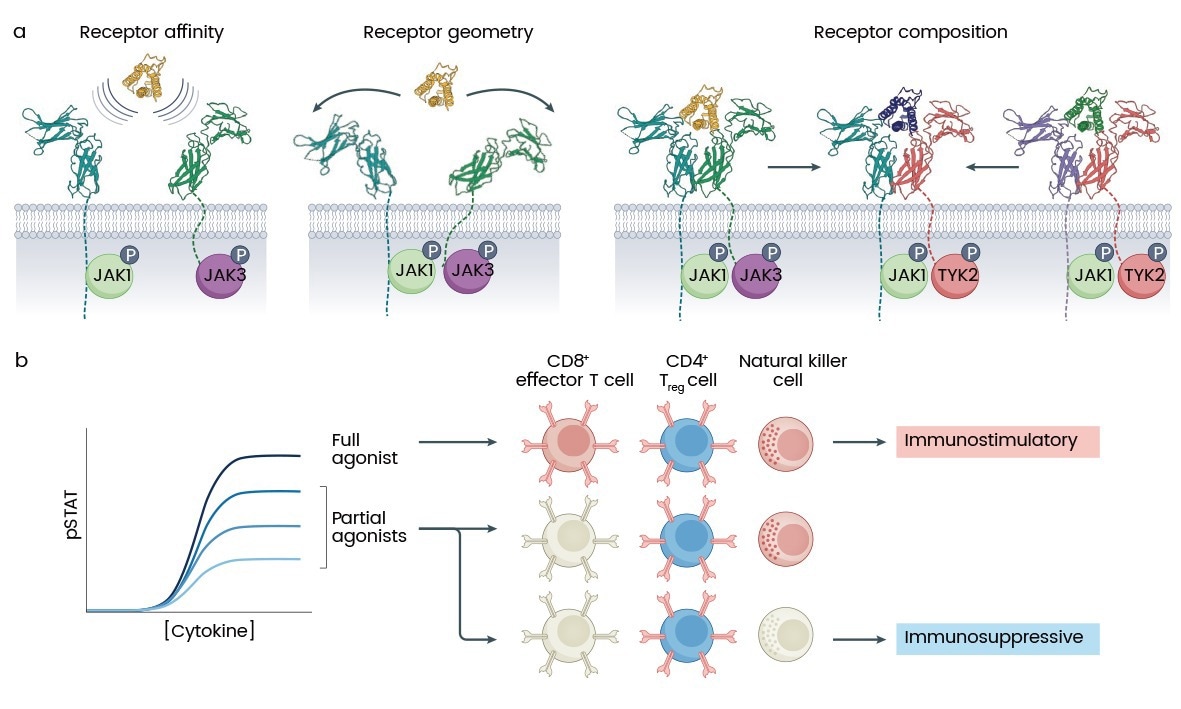
Figure 2. Tunable parameters in cytokine receptor pharmacology. (a) Model showing three controllable parameters in cytokine receptor signaling: receptor affinity, geometry/orientation, and subunit composition. (b) Cytokine partial agonists, with lower maximal signaling strength (Emax), can overcome pleiotropy by exploiting different signaling thresholds across cell populations. For example, IL-2 partial agonists can selectively expand CD4+ regulatory T cells (Treg cells) (blue) over effector CD8+ T cells or natural killer cells (red). JAK, Janus kinase; pSTAT, STAT phosphorylation4. (Source: https://doi.org/10.1038/s41573-022-00557-6)
Sino Biological’s support for organoid and stem cell research
Sino Biological provides a complete spectrum of recombinant cytokines and growth factors for stem cell and organoid cultures, improving research in drug discovery and regenerative medicine alike.
Their products undergo strict quality control and comprehensive validation to guarantee high purity, biological activity, stability, and low endotoxin levels. Their GMP-grade cytokines, built with a GMP quality management system, provide improved stability and quality. Moreover, Sino Biological offers diverse research reagents, such as small molecules, marker antibodies, ELISA kits, and beyond, to meet diverse research needs.
References and further reading
- Clevers, H. (2016). Modeling Development and Disease with Organoids. Cell, (online) 165(7), pp.1586–1597. https://doi.org/10.1016/j.cell.2016.05.082.
- Liu, F., et al. (2016). Drug Discovery via Human-Derived Stem Cell Organoids. Frontiers in Pharmacology, 7. https://doi.org/10.3389/fphar.2016.00334.
- Takahashi, K. and Yamanaka, S. (2006). Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell, (online) 126(4), pp.663–676. Available at: https://pubmed.ncbi.nlm.nih.gov/16904174/.
- Saxton, R.A., Glassman, C.R. and K Christopher Garcia (2022). Emerging principles of cytokine pharmacology and therapeutics. Nature Reviews Drug Discovery, 22(1), pp.21–37. https://doi.org/10.1038/s41573-022-00557-6.
- Yu, J., et al. (2007). Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science, (online) 318(5858), pp.1917–1920. https://doi.org/10.1126/science.1151526.
- Williams, L.A., Davis-Dusenbery, B.N. and Eggan, K.C. (2012). SnapShot: Directed Differentiation of Pluripotent Stem Cells. Cell, 149(5), pp.1174-1174.e1. https://doi.org/10.1016/j.cell.2012.05.015.
- Thermo Fisher Scientific Inc. (2024). ESC and iPSC differentiation pathways (poster). COL12197 0723.
- Lancaster, M.A. and Knoblich, J.A. (2014). Organogenesis in a dish: Modeling development and disease using organoid technologies. Science, (online) 345(6194), pp.1247125–1247125. https://doi.org/10.1126/science.1247125.
- Barker, N., et al. (2010). Lgr5+ve Stem Cells Drive Self-Renewal in the Stomach and Build Long-Lived Gastric Units In Vitro. Cell Stem Cell, 6(1), pp.25–36. https://doi.org/10.1016/j.stem.2009.11.013.
- Thorne, C.A., et al. (2018). Enteroid Monolayers Reveal an Autonomous WNT and BMP Circuit Controlling Intestinal Epithelial Growth and Organization. Developmental Cell, 44(5), pp.624-633.e4. https://doi.org/10.1016/j.devcel.2018.01.024.
- Nwokoye, P.N. and Abilez, O.J. (2024). Bioengineering methods for vascularizing organoids. Cell Reports Methods, (online) 4(6), p.100779. https://doi.org/10.1016/j.crmeth.2024.100779.
- Hirano, T. (2020). IL-6 in inflammation, autoimmunity and cancer. International Immunology, (online) 33(3), pp.127–148. https://doi.org/10.1093/intimm/dxaa078.
- Huang, H., et al. (2024). The functions and applications of organoids in rheumatic immune diseases. Journal of Holistic Integrative Pharmacy, (online) 5(2), pp.141–147. https://doi.org/10.1016/j.jhip.2024.06.004.
- Freedman, B.S., et al. (2015). Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nature Communications, (online) 6(1), pp.1–13. https://doi.org/10.1038/ncomms9715.
- Fu, Y., Tang, R. and Zhao, X. (2023). Engineering cytokines for cancer immunotherapy: a systematic review. Frontiers in Immunology, 14. https://doi.org/10.3389/fimmu.2023.1218082.
- Obeid, D.A., et al. (2024). Using Liver Organoids as Models to Study the Pathobiology of Rare Liver Diseases. Biomedicines, (online) 12(2), p.446. https://doi.org/10.3390/biomedicines12020446.
- Rabata, A., et al. (2020). 3D Cell Culture Models Demonstrate a Role for FGF and WNT Signaling in Regulation of Lung Epithelial Cell Fate and Morphogenesis. Frontiers in Cell and Developmental Biology, 8. https://doi.org/10.3389/fcell.2020.00574.
- Han, Y., et al. (2022). Human organoid models to study SARS-CoV-2 infection. Nature Methods, (online) 19(4), pp.418–428. https://doi.org/10.1038/s41592-022-01453-y.
About Sino Biological Inc.
Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.