Over the last few decades, worldwide life expectancy has risen. Longer life expectancy, on the other hand, is linked to an increased risk of neurodegenerative disorders like Alzheimer’s disease (AD) and Parkinson’s (PD).
Neurodegenerative diseases afflict almost 50 million people globally, with an estimated 115 million people expected to be affected by 2050. Patients with AD and PD become more debilitated and thus require 24-hour care.
Although there are presently no effective therapies to slow or halt the course of AD or PD, there is a large body of research underway to better understand the etiology and pathophysiology of these disorders. This effort, if continued, will eventually lead to the creation of novel medicines and cures.
In the search for therapeutics, it is vital to understand crucial players like amyloid precursor protein (APP) in AD and α-Syn in PD. The following article focuses on the current level of knowledge on significant protein targets in different neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease.
The most frequent neurodegenerative condition is Alzheimer’s disease. It is marked by gradual cognitive and behavioral deficits that eventually lead to dementia.
Extracellular amyloid plaques made up of amyloid-beta (Aβ) peptides and intracellular neurofibrillary tangles made up of hyperphosphorylated tau proteins are pathological hallmarks of Alzheimer’s disease (Figure 1).
Through a sequence of cleavages by β- and γ-secretases, a succession of 39–43 amino acid-containing Aβ peptides is generated from APP. These Aβs, especially Aβ42, are prone to forming dimers, oligomers, and insoluble fibril aggregates, which eventually form amyloid plaques.
Aβ42 is more typically seen in Alzheimer’s plaques than Aβ40. Total tau (t-tau) and phosphorylated tau (p-tau) are three generally accepted AD biomarkers, along with Aβ42.
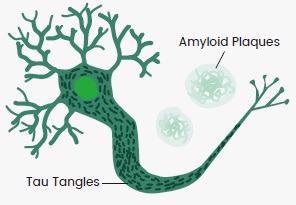
Figure 1. Abnormal Accumulation of Amyloid Beta (Aβ) and Tau. Image Credit: Sino Biological Inc.
Table 1. Development Status of Partial AD Drugs. Source: Sino Biological Inc.
| Drug |
Target |
Company |
Status |
| Aducanumab |
Amyloid beta |
Biogen |
FDA approval in 2021 |
| Solanezumab |
Amyloid beta |
Eli Lilly |
Phase 3 |
| Semorinemab |
tau |
Roche |
Phase 3 |
| Verubecestat |
BACE1 |
Merck |
Phase 3 |
| Semagacestat |
Gamma secretase |
Eli Lilly |
Phase 3 |
Many drug discovery efforts have been made in recent years to reduce or prevent AD progression by preventing the creation of Aβs and the plaques that come from them (Table 1). Solanezumab is a humanized monoclonal antibody that increases Aβ clearance by targeting the Aβ peptide’s mid-domain (Aβ13-28).
In Phase 3 of the clinical trial, however, Eli Lilly announced in 2016 that the medicine failed to halt cognitive deterioration.
Roche’s semorinemab, a human immunoglobin G (IgG) 4 antibody meant to target the tau protein’s N-terminal region and inhibit its spread between neurons, similarly failed in 2020.
A succession of clinical trial failures with anti-Aβ and anti-tau monoclonal antibodies has cast doubt on this method, prompting researchers to look for other targets, including the β-site APP cleaving enzymes (BACE) 1 and 2, and gamma-secretase.
As they convert APP into neurotoxic Aβ peptides, BACE1 and BACE2 are small-molecule therapeutic targets for Alzheimer’s disease. Modulating the function of these proteins has been a recent focus of AD medication development.
Sino Biological created premium recombinant target proteins and primary antibodies covering APP, BACE1 and BACE2 to aid drug development for Alzheimer’s disease. Please see Figure 2 for a limited product list.
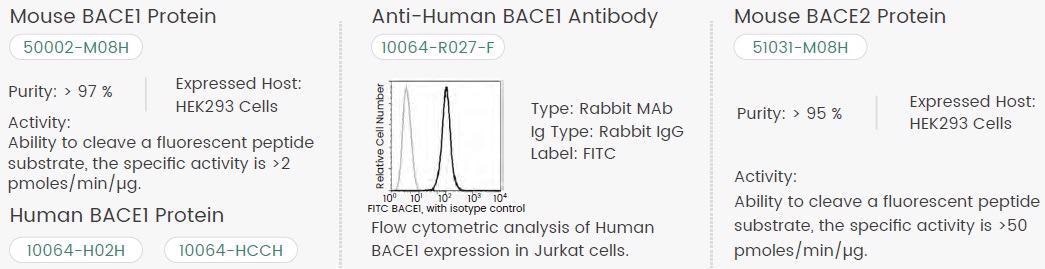
Figure 2. Featured Sino Biological BACE1 and BACE2 Pro. Image Credit: Sino Biological Inc.
PD is the second most prevalent neurodegenerative ailment after Alzheimer’s disease. The degeneration of dopaminergic neurons in the substantia nigra, and also Lewy bodies, and Lewy neurites made up of alpha-synuclein (α-Syn), are all symptoms of Parkinson’s disease.
α-Syn is widely and ubiquitously expressed throughout the brain, mainly inside neuronal presynaptic areas, as represented by the SNCA gene.
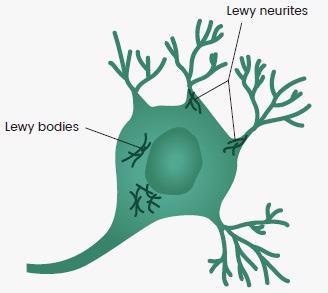
Figure 3. Lewy Bodies and Lewy Neurites in the Brain. Image Credit: Sino Biological Inc.
The synuclein family also comprises β- and γ-synuclein, in addition to α-Syn. PD is strongly linked to point mutations, duplications, and even triplication mutations in the SNCA gene.
α-Syn is being tested as a possible therapeutic target for the development of PD-modifying drugs, with different anti-α-Syn monoclonal antibodies. In May 2021, Roche began Phase 2b trials for prasinezumab, a humanized IgG1 monoclonal antibody targeting aggregated α-Syn.
Another successful technique for reducing α-Syn buildup and improving motor and cognitive function is α-Syn immunization.
An investigational vaccination called Affitope PD01A is a synthetic peptide that replicates the C-terminal residues of α-Syn (110–130). Immunization with Affitope PD01A decreased the accumulation of α-Syn in the substantia nigra and tyrosine hydroxylase-positive fibers in mice striatum.
Glutamate receptors, which play essential roles in controlling neuronal excitability, neurotransmitter release, and long-term synaptic plasticity, are another interesting target for PD therapy.
In animal models of Parkinson’s disease, mGluR 5 antagonists, as well as group II mGluR and mGluR4 agonists, have shown pharmacological efficacy. Molecular chaperones, SV2C, and LRRK2 have all been identified as new PD therapeutic targets. Autophagy-lysosomal pathways have also attracted attention as a possible treatment target for Parkinson’s disease.
Sino Biological’s high-quality α-and β-Syn proteins and antibodies for PD drug development are shown in Figure 4.
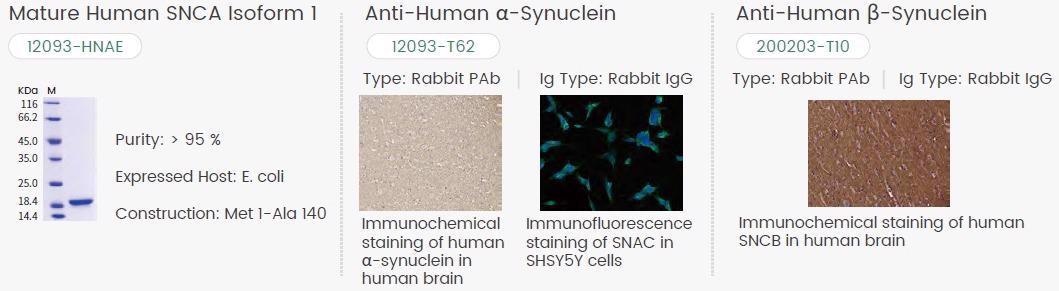
Figure 4. Featured Sino Biological α-and β-Synuclein Products. Image Credit: Sino Biological Inc.
Conclusion
Discovering safe and effective therapeutics for neurodegenerative disorders has proven difficult for drug discovery experts due to complicated and poorly understood molecular pathways. Despite this, research into the causes of neurodegenerative diseases and potential treatment techniques continues.
Monoclonal antibodies, oligonucleotides and cell therapies are among the treatments being tested in preclinical or clinical trials. These efforts will provide significant potential for the slowed progression, prevention and eventual treatment of these deadly diseases.
References
- DOI: 10.1176/appi.focus.20160030
- DOI: 10.3389/fnagi.2021.631770
- DOI: 10.1016/j.cca.2016.05.014
- DOI: 10.3233/JAD-122176
- DOI: 10.1186/s40035-022-00292-3
- DOI: 10.1111/j.1460-9568.2009.06873.x
- DOI: 10.3389/fphar.2020.00356
- DOI: 10.4103/1673-5374.306066
- DOI: 10.1186/s13024-019-0329-1
- DOI: 10.1124/pharmrev.120.000133
- DOI: 10.1007/s00401-014-1256-4
- DOI: 10.3390/ijms20184391
- DOI: 10.1038/nrd3802
- DOI: 10.1016/j.drudis.2022.01.016s
About Sino Biological Inc.

Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offers pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.