Cancer is a disease that comes under the category of one of the most challenging diseases. Numerous cancer treatments, like radiotherapy, surgery, immunotherapy and chemotherapy are available, but they often lead to tumor recurrence, suboptimal efficacy and therapy resistance.1,2
Stem cells, however, exhibit special characteristics, such as a high capacity for differentiation and self-renewal.
Stem cells have been studied extensively and can be approximately classified into various groups: Embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), mesenchymal stem cells (MSCs), hematopoietic stem cells (HSCs), Cancer Stem Cells (CSCs) and neural stem cells (NSCs)
In recent times, stem cells have shown to have the ability to enhance cancer treatment by regenerating cells following targeting cancer pathways, heavy therapy, thereby producing immune cells and being therapeutic carriers (Figure 1).3
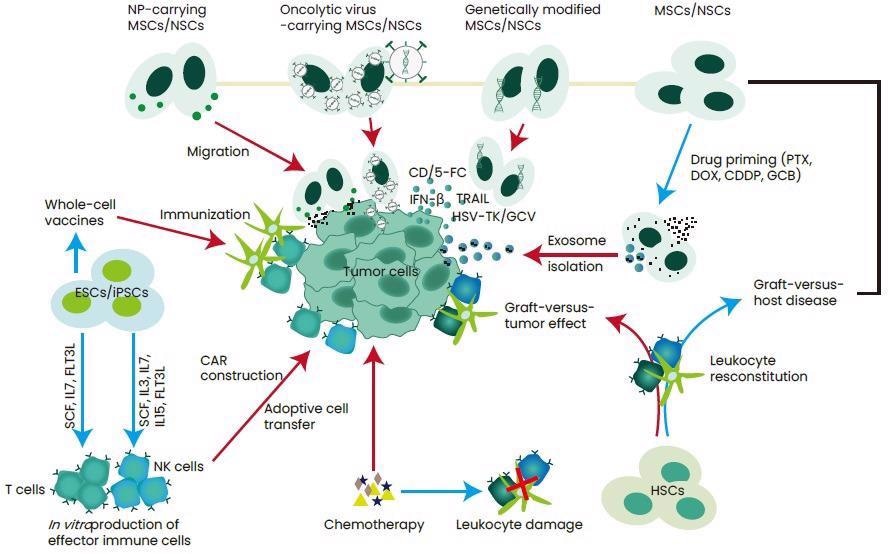
Figure 1. Strategies for the application of stem cell therapy in the treatment of cancer. Image Credit: https://doi.org/10.3390/cells9030563.
Stem cell transplantation
Hematopoietic stem cells (HSCs), situated in bone marrow, can develop all mature blood cells in the body. High-dose radiotherapy or chemotherapy not just has impacts on cancer cells but also on normal cells, thereby resulting in slow cell growth or also cell death.4
Currently, HSC transplantation is a standard treatment that has been utilized in multiple leukemia, myeloma and lymphomas targets following rounds of therapy to help patients recover from the disease.
For instance, in Leukemia patients, HSCs are being infused to help proliferate and distinguish into new blood cells by the addition of colony-stimulating factors (cat#: 10015-H07H), which trigger intracellular signaling pathways in HSCs.5
So far, the infusion of HSCs is considered the sole procedure for stem cells approved by the FDA. But the occurrence of graft-versus-host-disease (GVHD) while utilizing allogeneic sources of HSCs is still a challenge, which is frequently treated with immunosuppressive drugs.6-8
Targeting CSC pathways for cancer therapy
Initially, cancer stem cells (CSCs) were identified in leukemia in 1994. These cells are produced by producing epigenetic mutations in normal stem cells or precursor or progenitor cells and discovered inside tumor tissues. The collapse of cancer treatment could be assigned to the CSCs’ characteristics.
They have the potential to produce tumors through self-renewal and differentiation into multiple cellular subtypes as normal stem cells. Even though the proportion of CSCs in tumor tissues seems to be very low, they add up to multiple tumor malignancies, like recurrence, heterogeneity, metastasis, multidrug resistance and radiation resistance.9
The surface markers, like CD166 (cat#: 10045-MM03-F), CD44 (cat#: 12211-MM02-A), Endoglin/CD105 (cat#: 10149-MM13-F), HER2/ERBB2 (cat#: 10004-MM07T-F), Lgr5 (cat#: 100687-T04), and EpCAM (cat#: 10694-H08H-B), are frequently utilized to determine CSCs obtained from highly heterogeneous cell types in tumors.10
The CSCs activities are controlled by pluripotent transcription factors, several intracellular signal pathways and extracellular factors, and these factors can be utilized as drug targets for treating cancer. Thus, targeting CSCs can offer hope for several types of solid tumors to be treated.
Stem cell source for the production of immune cells
Natural killer (NK) cells and Chimeric antigen receptor (CAR) T cells have been successfully employed for anticancer immunotherapy. These cells are frequently generated from the patient, but the quantity and quality are difficult to control in vivo.
Outsourcing to induced embryonic stem cells (ESCs) and pluripotent stem cells (iPSCs) could provide limitless sources and allow the expansion of this immunotherapy to several patients.11
ESCs are separated from embryos, whereas iPSCs are induced from somatic cells in culture by overexpressing Yamanaka factors: Oct3/4 (cat#: HG13137), Sox2 (cat#: MG57011-UT), Klf4 (cat: HG12321-UT), c-Myc (cat#: HG11346). Incubation of ESCs and iPSCs in the growth medium consisting of NK cell or T cell-initiating cytokines.
For instance, stem cell factor (SCF, Cat#: 10451-H08B), IL-3 (cat#: 11858-HNAE), IL-7 (cat#: 50217-MNAE), IL-15 (cat#: 10360-HNCE) and FMS-like tyrosine kinase receptor-3 ligand (cat#: 10315-H07B) encourages differentiation of the immune cell.
High bio-activity recombinant IL-15
Cell proliferation assay has been conducted with the help of MO7e human megakaryocytic leukemic cells.
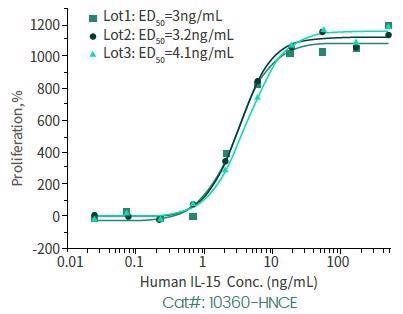
Human IL-15, N-cleavage. Image Credit: Sino Biological Inc
Stem cells as potential therapeutic carriers
Tumors are known as chronic wound tissue and their microenvironment, which constitutes secreted paracrine factors and extracellular matrix (ECM). This can draw attention to the migration of Mesenchymal stem cells (MSCs).12
MSCs are considered multipotent adult stem cells that exist in multiple tissues, such as the fat tissue, bone marrow and umbilical cord.
Numerous immune-related cytokines are called chemoattractants for MSCs to prostate cancer, multiple myeloma, osteosarcoma, and breast cancer cells, for instance, CXCL16 (cat#: 50142-M08H), SDF-1 (cat#: 10118-HNAE), CCL-25 (cat#: HG10601-M), TNF-α (cat#: 10602-HNAE), IL-1β (cat#: 10139-HNAE), and IL-6 (cat#: 10395-HNAE).13
Thus, MSCs could be used as possible carriers providing therapeutic agents in treating cancers
Featured recombinant TNF-α protein
Sino Biological has come up with bioactive recombinant TNF-α cytokines from several species, like humans, cynomolgus, mouse, ferret, rat and canines.
- High-purity
- Low-endotoxin
- Tag-free
- HPLC-verified
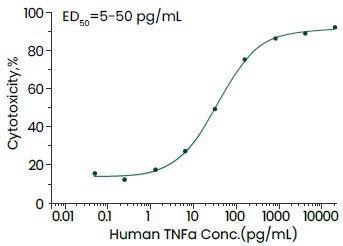
Cytotoxicity assay using L929 mouse fibrosarcoma cells in the presence of the metabolic inhibitor actinomycin D. Image Credit: Sino Biological Inc
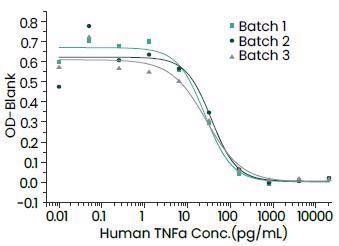
Batch-to-batch Consistency. Image Credit: Sino Biological Inc
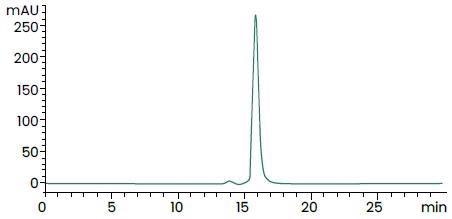
>95% as determined by SEC–HPLC. Image Credit: Sino Biological Inc
Featured recombinant IL-1β Protein
In comparison to the competing product, Sino Biological’s IL-1β protein illustrates greater bioactivity as displayed below.
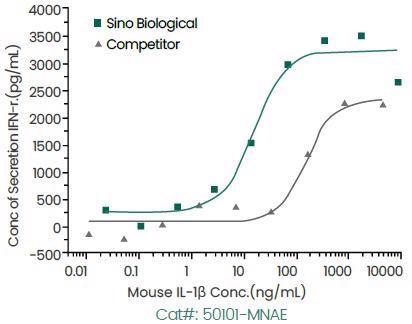
Induce Interferon Gamma Secretion. Image Credit: Sino Biological Inc
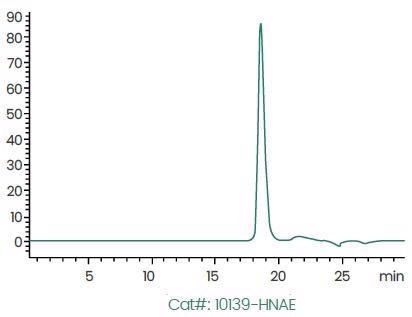
>95% Purity Determined by SEC–HPLC. Image Credit: Sino Biological Inc
MSCs have the potential to discharge nano-sized exosomes, known as a kind of extracellular vesicles (EV), consisting of several biological materials, to control cell-cell interaction.14
Anti-tumor siRNAs, mRNA or small-molecule drugs were successful in packing into MSCs and aim at the tumor niche to silence tumor-related genes or improve anti-tumor effects.15
Furthermore, MSCs can be used to carry anti-cancer drugs through nanoparticles (NPs).
MSCs-carried PLA NPs were successful in targeting brain tumors and paclitaxel-loaded NPs inhibited lung tumor growth in mice.16 Also, cancer cells could be killed selectively through oncolytic Viruses (OVs), while the naked OVs are simple to eliminate using immune cells.
Stem cells, similar to neural stem cells (NSCs), can be hopeful carriers to safeguard and provide OVs to tumor sites.17 NSCs, which are originally present in the central nervous system, have the ability to self-renew and produce new glial cells and neurons.
An early study discovered that OVs carried by human NSCs jointly with ionizing radiation and temozolomide could improve cytotoxicity to glioma tumor cells in vitro and increase the survival time of glioblastoma multiforme (GBM)-bearing mice.18
However, both MSCs and NSCs seem to be simpler to engineer to express various genes. This is accountable for the transformation of a prodrug into cytotoxic metabolites toward tumor cells.19 This gene therapy is known as “suicide gene therapy”.
Two clinical trials were completed in phase I. One made use of cytosine deaminase (CD)-expressing NSCs to transform 5-fluorocytosine (5-FC) into tumor-toxic 5-fluorouracil (5-FU) [NCT01172964, completed].
Another transformed ganciclovir from monophosphorylate to triphosphate form, which is highly cytotoxic, by Herpes simplex virus thymidine kinase (HSV-TK) which expresses MSCs [EudraCT 2012-003741-15, completed].
But the anti-tumor efficacy depends on dose control, various stem cells localized into the tumor microenvironment, and retention in tumor sites. In this mini-review, Sino Biological has initiated stem-cell-related cancer treatments.
Even though positive outcomes have been achieved by stem cell therapy, there are still a few side effects that have to be taken into account, for instance, the transformation of normal stem cells into cancer stem cells, the chronic GVHD following allogeneic HSC transplantation, the increased immune response, etc.
In brief, stem cell technologies exhibit high potential for tumor treatments but it still requires additional measures to overcome the difficulties before they could make their way into clinical trials.
References
- Vanneman, M, Dranoff, G (2012) Combining immunotherapy and targeted therapies in cancer treatment. Nature Reviews Cancer, 12, 237–251.
- Wang X, Zhang H, Chen X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist, 2019;2:141-160.
- Chu DT, Nguyen TT, Tien NLB, et al Recent Progress of Stem Cell Therapy in Cancer Treatment: Molecular Mechanisms and Potential Applications. Cells, 2020;9(3):563. Published 2020 Feb 28.
- Baskar R, Dai J, Wenlong N, Yeo R, Yeoh KW. Biological response of cancer cells to radiation treatment. Frontiers in Molecular Biosciences; 1:24. Published 2014 Nov 17.
- Hopman RK, DiPersio JF. Advances in stem cell mobilization. Blood Reviews, 2014;28(1):31-40.
- Copelan, E.A. Hematopoietic Stem-Cell Transplantation. The New England Journal of Medicine, 354, 1813–1826.
- Méndez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; MacArthur, B.; Lira, S.A.; Scadden, D.T.; Ma’Ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and hematopoietic stem cells form a unique bone marrow niche. Nature, 466, 829–834.
- Lee, R.H.; Oh, J.Y.; Choi, H.; Bazhanov, N. Therapeutic factors secreted by mesenchymal stromal cells and tissue repair. Journal of Cellular Biochemistry, 2011, 112, 3073–3078.
- Yu Z, Pestell TG, Lisanti MP, Pestell RG. Cancer stem cells. The International Journal of Biochemistry & Cell Biology. 2012;44(12):2144-2151
- Codd, A.S.; Kanaseki, T.; Torigo, T.; Tabi, Z. Cancer stem cells as targets for immunotherapy. Immunology, 153, 304–314
- Iriguchi, S.; Kaneko, S. Toward the development of true “off-the-shelf” synthetic T-cell immunotherapy. Cancer Science, 2019, 110, 16–22.
- Aravindhan, S., Ejam, S.S., Lafta, M.H. et al. Mesenchymal stem cells and cancer therapy: insights into targeting the tumour vasculature. Cancer Cell International. 2021, 21, 158.
- Jung, Y.; Kim, J.K.; Shiozawa, Y.; Wang, J.; Mishra, A.; Joseph, J.; Berry, J.E.; McGee, S.; Lee, E.; Sun, H.; et al.Recruitment of mesenchymal stem cells into prostate tumors promotes metastasis. Nature Communications, 2013, 4,1795
- Fuhrmann, G.; Serio, A.; Mazo, M.M.; Nair, R.; Stevens, M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. Journal of Controlled Release, 2015, 205, 35–44
- Kooijmans, S.A.; Schiffelers, R.M.; Zarovni, N.; Vago, R. Modulation of tissue tropism and biological activity of exosomes and other extracellular vesicles: New nanotools for cancer treatment. Pharmacological Research, 2016, 111, 487–500.
- Pascucci, L.; Coccè, V.; Bonomi, A.; Ami, D.; Ceccarelli, P.; Ciusani, E.; Viganò, L.; Locatelli, A.; Sisto, F.; Doglia, S.M.; et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. Journal of Control Release, 192, 262–270.
- Marelli, G.; Howells, A.; Lemoine, N.R.; Wang, Y. Oncolytic Viral Therapy and the Immune System: A Double-Edged Sword against Cancer. Frontiers in Immunology. 2018, 9, 866.
- Duebgen, M.; Martinez-Quintanilla, J.; Tamura, K.; Hingtgen, S.; Redjal, N.; Shah, K.; Wakimoto, H. Stem Cells Loaded With Multimechanistic Oncolytic Herpes Simplex Virus Variants for Brain Tumor Therapy. Journal of the National Cancer Institute, 2014, 106
- Sage, E.; Thakrar, R.M.; Janes, S.M. Genetically modified mesenchymal stromal cells in cancer therapy. Cytotherapy, 2016, 18, 1435–1445.
About Sino Biological Inc.

Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offers pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.