The concept of a ‘plasmid’ was first introduced by Joshua Lederberg in 1952. Since that time, it has become widely recognized as a circular DNA able to viably function as a carrier for introducing a foreign gene into a specific host cell.
Following the discovery of a range of plasmid systems, it has become possible to design and develop advanced recombinant protein expression technology. This has in turn opened up the possibility to scale up the production of many proteins of interest which are generally only present in small numbers in their natural environments.
The ability to select the expression host is also a central factor in facilitating the production of the most biologically relevant recombinant proteins.
E.coli is generally regarded as a ‘workhorse’ for recombinant protein expression, but it offers limited protein folding capacity and cannot add suitable post-translational modifications onto target proteins.
Advanced eukaryote cells have been developed to meet these needs, including yeast, insect cells, and mammalian cells (HEK293 and CHO). Each of these systems offers specific benefits and limitations, however (Table 1).
Table 1. Commonly used host cells for recombinant protein expression. Source: Sino Biological Inc.
| Expression Host |
E.coli |
Yeast |
Insect |
Mammalian |
| Category |
Prokaryote |
Eukaryote |
Eukaryote |
Eukaryote |
| Culture density |
High |
High |
High |
Medium |
| Culture duration |
Short
(1~2 days) |
Short
(1~2 days) |
Medium
(2~4 days) |
Long
(5~7 days) |
| Protein folding |
Limited |
Yes |
Yes |
Yes |
| PTM |
None |
Glycosylation |
Glycosylation, phosphorylation… |
Glycosylation, phosphorylation… |
Suitable
proteins |
Proteins with
low MW |
Secreted,
intracellular |
Secreted,
intracellular |
Secreted |
| Cost |
Low |
Low |
High |
High |
| Example Cell Lines |
BL21(DE3), Rosetta… |
pichia pastoris |
sf9, sf21, High-five |
HEK293, CHO |
| Note |
Inclusion
bodies |
High mannose glycan |
Low MW glycan |
|
Insect cell mediated recombinant protein expression
Insect cells necessitate the use of an intermediate – baculovirus –to enable protein expression. Baculoviruses are a varied group of DNA viruses able to infect more than 600 types of insect cells. This makes them an ideal vehicle for the introduction of a target gene into a specific host cell.
Autographa californica multiple nucleopolyhedrovirus (AcMNPV) is regarded as the most ideal baculovirus for this purpose. Figure 1 shows a summary flow chart of its characterization process.
This process involves a gene encoding the protein-of-interest being inserted into a primary vector. This is then cloned into a secondary vector referred to as a ‘Bacmid.’
Next, the Bacmid is transferred into an appropriate bacteria strain - typically E.coli –to facilitate initial virus production and assembly. This results in the production of a Generation 1 baculovirus (P1).
The P1 virus is amplified in an insect cell (for example, sf9), allowing this to reach a suitable titer (P2). The P2 virus can be employed in the infection of either an identical or different insect cell line for protein expression, for example, High-five.
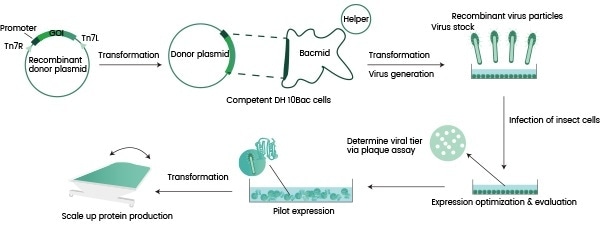
Figure 1. Flowchart for recombinant protein expression in insect cells. Image Credit: Sino Biological Inc.
Application notes and processes
Insect cells are highly flexible and adaptable expression hosts suitable for an array of recombinant proteins. Most notably, their robust folding capabilities and comparatively high culture density make them well suited to the expression of complex virus proteins and intracellular protein.
The HPV vaccine Cervarix was produced via an insect cell line. This vaccine was made available in the format of virus-like particles (VLPs) and approved for human use in 2007.
Highly active proteins produced in insect cells offer excellent potential for therapeutic and vaccine development. These proteins see routine use in a wide range of disciplines from biophysics and biochemistry for structure elucidation through to drug design, diagnostic reagent development and assay establishment.
Sino Biological extensively employs insect cells for recombinant protein product development and associated contracted research projects. This article outlines a series of application notes and case studies which aim to showcase the versatility and power of the insect cell expression system.
In terms of the ideal cell line, Sino Biological routinely uses sf9 as a virus amplifier and High-five as the production cell line – the latter typically offering relatively higher protein expression than its counterparts.
The company has also developed the proprietary SCD6SF culture medium for insect cell culture. This culture is currently only available in mainland China, however. The culture is typically maintained at approximately 6x106 cells/mL when working with small-scale protein expressions.
Insect cells can be easily adapted to work with signal peptides from different species. Signal peptides from both mammals and viruses can be utilized to guide protein secretion in insect cells, though signal peptides from other species are often less accommodated, for example, bacteria and aquatic creatures.
Insect secretion signal peptides offer a potential alternative in these cases, including honeybee melittin signal peptide, gp64 and gp67.
Working with intracellular proteins typically involves cell lysis and protein extraction protocols. These methods play key roles in stabilizing the target protein ahead of purification.
When working with a specific protein, it is advisable to conduct a pilot study to establish the most appropriate extraction protocol. Example protocol considerations include:
- Cell lysate duration and method
- Lysate buffer recipe
- Extraction buffer pH
- Buffer component, for example, PBS, Tris or HEPES
- Salt concentration
- Detergent type and concentration
It is also prudent to perform a small-scale purification test using the designated method to evaluate the purity and yield of the final protein product.
Example case studies
Protein construct
A number of structural features on a target protein may lead to instability in the recombinant protein expression; for example, regions exhibiting high levels of hydrophobicity, highly disordered errors and repetitive amino acid motifs.
In the example shown in Figure 2, these regions are not directly involved in protein function, so it is possible to remove these to enhance protein expression.
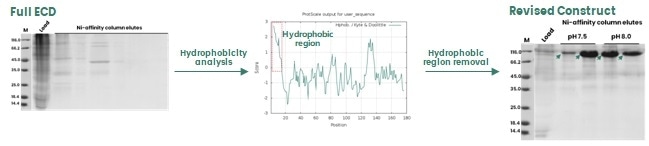
Figure 2. Removal of a hydrophobic region in the protein sequence to enhance protein expression in insect cells. Image Credit: Sino Biological Inc.
Purification procedure
Proteins are extremely sensitive to their surrounding chemical environment, and their stability is significantly affected by changes in pH, ionic strength and oxidative status.
Additives may be required during purification to stabilize the target protein or help enable tag exposure. In the example shown in Figure 3, the target protein (a single-pass transmembrane protein) was not adequately extracted via the use of the original detergent formula (1), most likely due to the inadequate His-tag exposure.
Adjusting the detergent formula (2) helped to facilitate enhanced protein extraction. During the final polishing step, a further detergent (DDM) was used to better stabilize the final protein product.
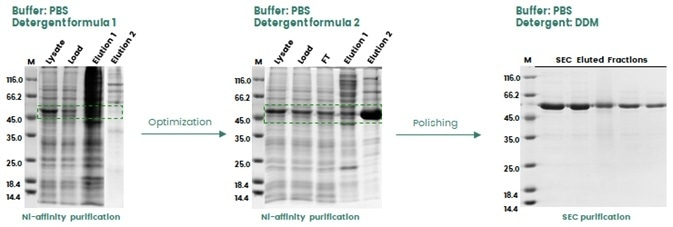
Figure 3. Buffer optimization during protein purification to enhance protein recovery in insect cells. Image Credit: Sino Biological Inc.
Recombinant proteins are one of the cornerstones of contemporary biologics development.
Insect cells represent an excellent choice of expression host. The cells offer correct protein folding and PTM, as well as being well suited to high-density cell culture. Insect cells can also produce secreted and intracellular proteins across a number of species.
Specific technical expertise – such as that available via Sino Biological - is required for advanced protein design, and it is necessary to use a systematic optimization approach to ensure the development of high-quality recombinant proteins via insect cells.
About Sino Biological Inc.

Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offers pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.