The emergence of antibody-drug conjugates (ADCs) has seen them become a promising class of anticancer therapeutics, exhibiting better precision and reduced off-target toxicity in targeting cancer cells.1
The basic mechanism involves monoclonal antibodies affixing themselves to overexpressed target antigens on cancer cells, resulting in internalization, lysosomal fusion, and subsequent release of cytotoxic payloads, provoking tumor cell death.2
Other mechanisms, such as antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and antibody-dependent cellular phagocytosis (ADCP), also promote a general anticancer effect.3
The development of ADCs involves a thorough review of several components, such as target-specific antibodies, cytotoxic payloads, and linkers.4 This article considers the structure, mechanism of action, and evolution of three generations, clinical success stories, challenges encountered, and future implications of ADCs.
Mechanism of action
ADCs target cancer cells with enhanced precision and limited off-target toxicity in comparison to traditional chemotherapy agents. The mechanism involves the binding of a monoclonal antibody to an overexpressed target antigen on cancer cells, which leads to internalization and formation of endosomes.
Lysosomal fusion provokes detachment of cytotoxic payloads that target specific subcellular structures, including DNA or microtubules, bringing about cell death. Cytotoxic payloads can also be implanted at the cell membrane and provoke a bystander effect, targeting any adjacent cells and assisting the overall process of tumor cell destruction.4
Moreover, mechanisms such as ADCC, CDC, and ADCP may boost the anticancer effect of ADCs. The Fab segment attaches itself to the antigen, while the Fc segment interacts with immune cells and elements of the complement system for direct cell death.5 Additionally, monoclonal antibodies can impede downstream signal transduction, as demonstrated by the blocking of HER2-related pathways for apoptosis by trastuzumab.4,5
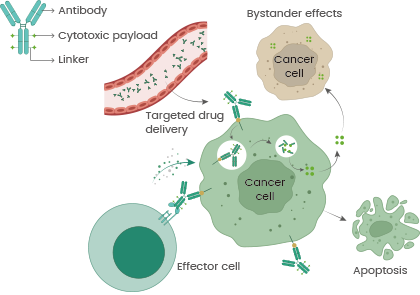
Figure 1. Mechanisms of Antibody-Drug Conjugates (ADCs) to selectively kill cancer cells. DOI: 10.1038/s41392-022-00947-7.
ADC building blocks
An ADC is made up of a target-specific mAb, a cytotoxic payload, and a chemically synthesized linker that covalently connects the toxin to the antibody.4,6,7 The mAb attaches to certain antigens on the tumor cell surface, and ADCs are internalized into tumor cells during the formation of antibody-antigen complexes.1,3
ADCs are usually transported from the endosome to the lysosome, where the linker is cleaved before a release of the small molecule cytotoxins, which triggers tumor cell death.1,4,7 Generally, ADC development necessitates an examination of the target antigens, antibodies, cytotoxic payload, and linker (Figure 2).
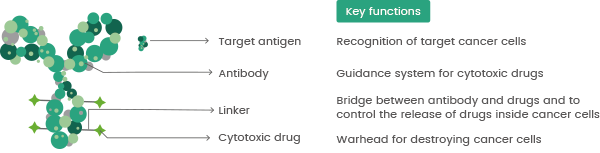
Figure 2. Basic components of The ADC include antibody, linker, and cytotoxic drug. Antibody targets binding, payload destroys cancer cells, and linker controls drug release. DOI:10.1016/j.biopha.2023.114408.
Antibodies
Non-secretory target antigens are required for ADCs, predominantly on tumor cell surfaces, and internalize post-binding for the efficient release of the cytotoxic payload into the tumor cells.8
Common ADC targets include HER2, Trop2, Nectin4, EGFR, CD19, CD22, CD33, CD30, BCMA, and CD79b.1,3,4,7,9
The ever-changing landscape includes emerging targets, such as EpCAM, CD70, CD25, CD166, all of which demonstrate promising results.10
Immunoglobulins (IgGs), specifically IgG1, are typically employed throughout clinical studies for ADCs.1 IgG1, found in rich supply in serum, induces robust effector functions, which include ADCC, ADCP, and CDC, as a result of the high Fc receptor binding affinity.1,3
Antibodies considered ideal for ADCs need high affinity, effective internalization, low immunogenicity, and a prolonged plasma half-life.3,7 Currently, ADCs mostly use fully humanized antibodies, limiting immunogenicity in contrast to earlier mouse antibodies.1,3,9
Featured hematological malignancy target protein from Sino Biological
Human CD22/Siglec-2 Protein, HPLC-verified (Cat#: 11958-H08H)
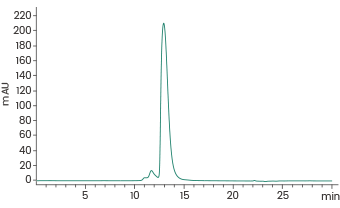
≥ 90 % as determined by SEC-HPLC. Image Credit: Sino Biological Inc
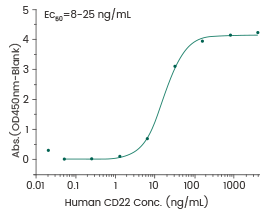
Binding assay: Immobilized anti-CD22 antibody can bind human Siglec-2/CD22 protein. Image Credit: Sino Biological Inc
Featured solid tumor target protein from Sino Biological
Human Mesothelin/MSLN Protein, HPLC-verified (Cat#: 13128-H08H1)
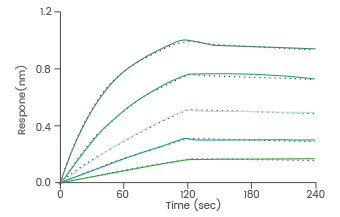
BLI assay: Loaded anti-MSLN antibody on ProA Biosensor can bind human Mesothelin protein. Image Credit: Sino Biological Inc
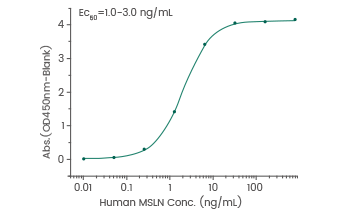
Binding assay: Immobilized anti-MSLN antibody can bind human MSLN/Mesothelin protein. Image Credit: Sino Biological Inc
Cytotoxic payload
No more than 2% of ADC administered reaches the tumors targeted.5 Therefore, sufficiently high potency (nano and picomolar IC50) is crucial for ADC payload compounds.5 Tubulin inhibitors and DNA damaging agents are among the current cytotoxic payloads.1,7,11
Cytotoxins considered well-suited for the task require high toxicity, low immunogenicity, and constancy, with functional groups that can be modified for mAb linkage.1 The drug-antibody ratio (DAR) is key here; low DAR may impede efficacy, while high DAR may bring about off-target toxicity as a result of instability in ADC development.1,4
Amongst the innovative approaches in payload design for ADCs, the exploration of non-toxic payloads, including RNA inhibitors, immuno-agonists (such as Toll-like receptors and STING agonists)1,4, and apoptosis-promoting Bcl-xL inhibitors1, all aim to improve anti-tumor immune responses, extend treatment windows, and enhance safety and efficacy.
Current developments include PROTAC (Proteolysis-targeting chimeras) molecules that intend to incorporate catalytic properties for the targeting of undruggable proteins.5 Moreover, the development of hybrid payloads that combine various mechanisms helps achieve synergistic effects while targeting several refractory cancers with heterogeneity and drug resistance.5
Linker
Cleavable and non-cleavable linkers can be leveraged to control the release of the cytotoxic payload.3,4 Cleavable linkers take advantage of the environmental differences between circulation and tumor cells, using chemical cleavage (hydrazone and disulfide bonds)4,9 or enzyme cleavage (glucuronide and peptide bonds).1,9
Hydrazone-linked ADCs are stable throughout circulation and release cytotoxic payloads in lysosomes (pH 4.8) and endosomes (pH 5.5–6.2) upon penetrating cancer cells.1 Non-cleavable linkers are reliant on lysosomal degradation of the complete structure while holding onto the charged amino acids in the payload.3,4,9
The linker's role is vital when it comes to ensuring efficient release within target tumor cells, as it helps prevent premature release in the plasma that could otherwise damage normal tissues.
Conjugation methods
Two types of conjugation techniques are typically used; stochastic and site-specific.1 Stochastic conjugations by amide pairing in ADCs, such as gemtuzumab ozogamicin, T-DM1, and inotuzumab ozogamicin, result in variable drug-antibody ratios (DAR 0–8) due to randomness among several of the lysine residues.1
This may lead to an unstable scenario, which can cause early payload release, off-target toxicity, and harm the chances of retaining consistency.3,7 Comparatively, site-specific conjugation is dependent on cysteine-based coupling, revealing disulfide bonds for a controlled approach and limiting any chance of heterogeneity.1,5 However, it can undermine antibody integrity.1
Evolution of ADCs
Since February 2024, the FDA has given 12 ADCs approval for several different cancers, including lymphomas, leukemia, myeloma, and solid tumors, such as HER2+ breast cancer, urothelial cancer, metastatic triple-negative breast cancer, and gastric cancer.
Amongst these ADCs are trastuzumab emtansine (T-DM1) for HER2-positive breast cancer and inotuzumab ozogamicin for acute lymphoblastic leukemia.
Moreover, there are more than a hundred ADC candidates in clinical trials, with a good number of them already showing great success in advanced phases.12 These success stories highlight the potential of ADCs to revolutionize the future of cancer treatment.12
Table 1. FDA Approved ADCs1,9 Source: Sino Biological Inc
| Approved ADC |
Target |
Indication |
Approved Year/ Company |
Payload |
Linker |
| Gemtuzumab Ozogamicin (Mylotarg®) |
CD33 |
Acute myeloid leukemia |
2000, 2017;
Pfizer |
N-acetyl-γ-calicheamicin |
Hydrazone |
| Brentuximab Vedotin (Adcetris®) |
CD30 |
Hodgkin lymphoma, systemic anaplastic large-cell lymphoma-cell lymphoma |
2011;
Seagen |
MMAE |
mc-VC-PABC |
| Trastuzumab Emtansine (Kadcyla®) |
HER-2 |
Breast cancer |
2013;
Roche |
DM1 |
SMCC |
| Inotuzumab Ozogamicin (Besponsa®) |
CD22 |
B-cell precursor acute lymphoblastic leukemia |
2017;
Pfizer |
N-acetyl-γ-calicheamicin |
Hydrazone |
| Moxetumomab pasudotox (Lumoxiti®) |
CD22 |
Hairy cell leukemia |
2018;
AstraZeneca |
mc-VC-PABC |
PE38 |
| Polatuzumab Vedotin (Polivy®) |
CD79b |
Diffuse large B-cell lymphoma |
2019;
Roche |
MMAE |
mc-VC-PABC |
| Enfortumab Vedotin (Padcev®) |
Nectin-4 |
Urothelial cancer |
2019;
Seagen |
MMAE |
mc-VC-PABC |
| Trastuzumab Deruxtecan (Enhertu®) |
HER-2 |
Breast cancer |
2019; Daiichi Sankyo |
DXd |
tetrapeptide |
| Sacituzumab Govitecan (Trodelvy®) |
Trop-2 |
Breast cancer, urothelial cancer |
2020;
Immunomedics |
SN38 |
CL2A |
| Belantamab Mafodotin (Blenrep®) |
BCMA |
Multiple myeloma |
2020, Withdrawal in 2022; GSK |
MMAF |
mc |
| Loncastuximab Tesirine (Zynlonta®) |
CD-19 |
Large B-cell lymphoma |
2021; ADC Therapeutics |
PBD dimer |
dipeptide |
| Tisotumab Vedotin (Tivdak®) |
Tissue factor |
Cervical Cancer |
2021; Genmab/Seagen |
MMAE |
mc-VC-PABC |
| Mirvetuximab Soravtansine (Elahere®) |
FRα |
Ovarian cancer |
2022;
ImmunoGen |
DM4 |
sulfo-SPDB |
Challenges and future directions
ADCs will still face challenges, such as complex pharmacokinetics, side effects, narrow tumor targeting, and drug resistance.4,7,9 Tumor penetration is impeded by ADCs' large molecular weight.1,4,5
To face these challenges head-on, next-generation ADCs are centered on the optimization of monoclonal antibodies, linkers, and payloads, leveraging bispecific antibody technology, and utilizing dual-payload ADCs.1,4,5
ADCs possessing a smaller molecular weight, including PEN-221, attempt to improve penetration efficiency, particularly in tumors that are typically challenging.1 Finally, another promising approach is the use of two cytotoxic agents as payloads to reduce ADC resistance.8
Sino Biological is leading the way in making great strides in ADC research with an extensive range of products and services. A diverse range of ADC target proteins are available across Sino Biological’s portfolio for precise cancer cell drug delivery, as well as a supporting cast of MMPs, Cathepsins, and uPA enzymes for optimal linker cleavage.
A range of high-quality FACS antibodies, reliable Fc receptor proteins, and specialized anti-payload antibodies can aid immune cell analysis and therapeutic development by assessing efficacy.
Beyond its products, Sino Biological’s services cover meticulous in vitro bioactivity validation, binding activity assays, internalization assay studies, neutralizing/blocking assays, anti-idiotypic antibodies development for PK/ADA assays, and control antibody production, all of which ensure a complete, well-rounded approach to ADC research.
About Sino Biological Inc.
Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.