Antibody drugs such as monoclonal antibodies (mAb), bispecific antibodies, antibody-drug conjugates (ADCs), and nanobodies garnered increased interest in research and development circles.
Sino Biological has developed a series of in vitro bioassay services to streamline antibody drug development projects. This scheme is due to the importance of in vitro screening and target validation during the early stages of antibody discovery.
Sino Biological offers a variety of reagents and an extensive range of in vitro efficacy evaluation services to meet the various trends and needs of testing practices.
Why in vitro bioactivity validation?
The bioactivity of mAbs is usually determined after the rigorous testing of efficacy and potency. This is a key component when establishing the antibody-drug evaluation system and takes up a central role in determining the mechanism of action (MOA) of drugs, offering a solid base when it comes to preparing investigational new drug (IND) submissions.
In vitro bioassays for antibodies can be grouped into two main categories:
- Antigen-binding assays include ELISA, flow cytometry, surface plasmon resonance (SPR), and biolayer interferometry (BLI).
- Biological activity assays include ADCC/ADCP/CDC, apoptosis, cytokine release, cell proliferation inhibition, cell killing activity, neutralization activity, and antibody internalization.
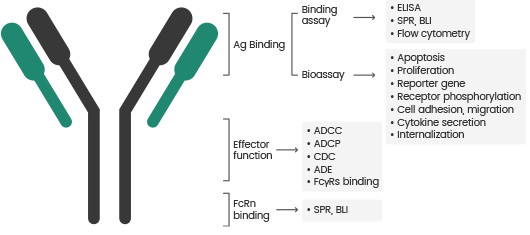
Bioactivity Evaluation Methods of Antibody Drugs. Image Credit: Sino Biological Inc.
CDC assay service
Complement-dependent cytotoxicity assay service
Complement-dependent cytotoxicity (CDC) in mAb-based therapy starts with binding the complement protein C1q to the Fc domain of mAbs for the opsonization of a target cell.
C1q binding triggers the complement cascade to initiate the membrane attack complex (MAC). This creates the pores that induce the lysis of the target cells.
Antibody-mediated CDC activity is crucial for developing and quality control (QC) antibodies. Sino Biological has developed a platform for testing the CDC activity of antibodies using rabbit complement.
Validation of the assay has been confirmed on a number of popular targets.
Service highlights
- Availability of well-defined application scenarios
- Comprehensive project experience
- High sensitivity and high signal-to-noise ratio
- Providing high-quality research-grade biosimilar control antibodies
- Suitable for antibody screening and quality control during drug lot release
- Validated by multiple targets such as PD-1, TIGIT, EGFR, and VEGF
Service details
Note: If there is a lack of positive control, please contact Sino Biological in advance.
Rabbit complement is typically used, if it is necessary to use other complements then the client needs to provide the relevant information.
ADE assay service
Antibody-dependent enhancement assay service
Antibody-dependent enhancement (ADE) mainly concerns a pathogen infection-induced body reaction.
In the process of pathogen infection, instead of stopping the virus from entering cells, the original neutralizing antibodies boost virus replication in the body and bring about significant pathological reactions by interacting with Fc receptors or complements to infect macrophages and granulocytes.
Sino Biological offers a range of professional ADE assay services, which enable biotherapeutic development. Sino can also provide cell line development services, tailored pseudovirus services, and other related products.
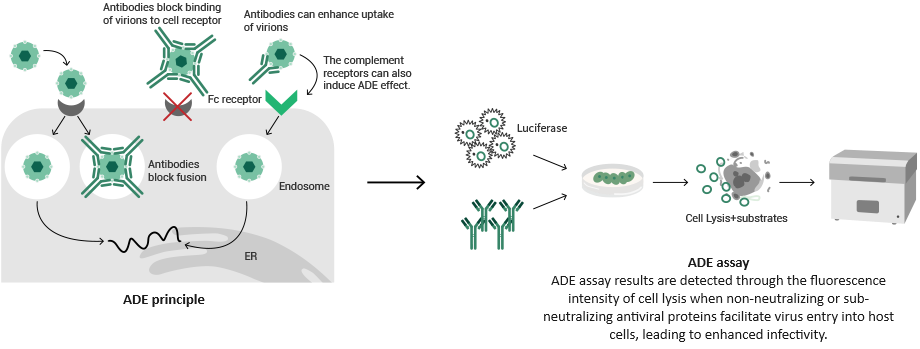
Image Credit: Sino Biological Inc.
Featured case study
A reporter gene assay was utilized to determine the ADE effect of antibodies. While no ADE effect was detected in the sample, increased fluorescence intensity signaled this effect was in the positive control.
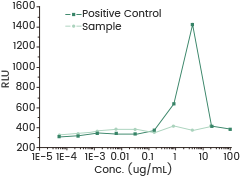
Measured by its ability to enhance viral entry into CHO-K1 cells induced by Fc-FcγRⅡ in a Luciferase receptor Assay System. Image Credit: Sino Biological Inc.
Antibody internalization assay service
Antibody-drug conjugates (ADCs) are now an important topic in antibody drug development. ADC encompasses antibody, linker, and cytotoxic drug components.
Once the antibody has bound to the target antigen on the cell surface of tumor cells, the ADC drug is endocytosed by the tumor cells and encapsulated within the intracellular lysosomes to release cytotoxic molecules that impair DNA or prevent the division of tumor cells, consequently killing the tumor cells.
Antibody internalization is a key element used to establish the bioactivity of ADCs. Sino Biological offers effective antibody internalization assay services to determine viable antibody candidates for ADC development.
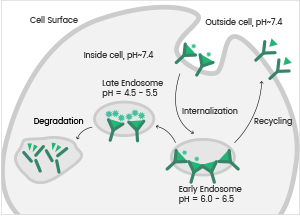
The Process of Antibody Internalization. Image Credit: Sino Biological Inc.
Service highlights
- Around 300 cell lines (tumor cell lines + modified cell lines) are available
- High signal-to-noise ratio with stable assay results (good reproducibility)
- Various assay platforms: flow cytometry, confocal platform, high-content analysis
- Validated by multiple targets such as TROP2, PD1, and Her2
Case study of TROP2 antibody internalization assay
Immunofluorescence results revealed that TROP2 antibody enters the intracellular space 1 h after being introduced to the cell culture. increased intracellular fluorescence signaled antibody internalization.
.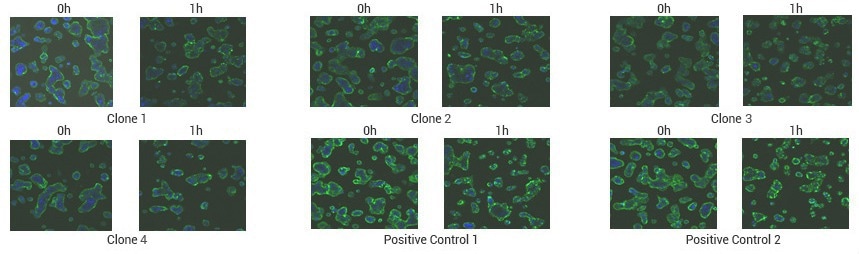
Image Credit: Sino Biological Inc.
Antibody-binding activity assay service
ELISA and flow cytometry are frequently used for assay binding. Sino Biological offers a range of professional antibody-binding bioassay services to support antibody drug discovery.
Sino Biological also provides products and services associated with target proteins, including both recombinant protein expression and cell line construction.
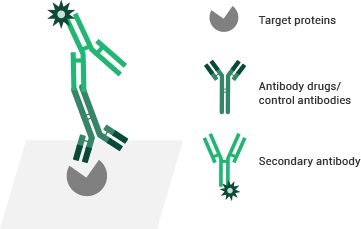
Testing principles of the ELISA binding assay. Image Credit: Sino Biological Inc.
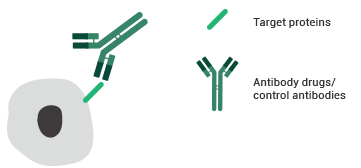
Testing principles of the FACS binding assay. Image Credit: Sino Biological Inc.
Service highlights
- Vast project experience
- 5000+ high-quality target proteins
- Well-defined applications are available
- “One-stop” services with the supply of complementary products
- Providing high-quality research-grade biosimilar control antibodies
- Standardized and customized services to meet individual requirements
Neutralizing/Blocking antibody validation service
Assessing the activity of neutralizing/blocking antibodies is a key factor in drug development. For instance, a great number of the immune checkpoints are provoked by ligand–receptor interactions; they can be easily impeded by antibodies or controlled by recombinant ligands or receptors.
Sino Biological can supply pseudovirus assays, immune checkpoints, and cytokine neutralizing/blocking antibody functional validation services, which includes both cell activity assay and neutralization titer assay.
A number of natural samples and a wealth of testing experience has allowed Sino Biological to conduct thorough and accurate method validation on kits and offer convenient "one-stop" ELISA kit development services.
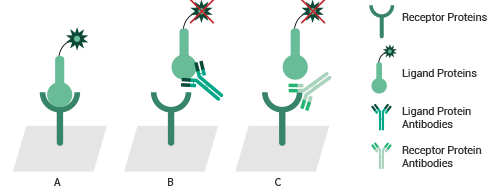
Test neutralizing/blocking activity of antibodies by ELISA-binding assay. A. In the absence of neutralizing antibodies, the ligand binds to the receptor and the signal is detected. B and C: After adding neutralizing antibodies, the ligand cannot bind to the receptor, so no signal is detected. Neutralizing antibodies can target either the receptor (B) or the ligand (C). Image Credit: Sino Biological Inc.
Service highlights
- Professional research and development solution with more than 10 years of project experience and screening over 100,000 ELISA antibodies
- Extensive resource of antibody targets, over 2200 targets and 30,000 antibody products, which provide a "one-stop" service for clients
- Enhanced quality assurance, with varied natural sample acquisition channels and testing experience
- "One-stop" service with short turnaround time and high efficiency, as well as convenient and fast ELISA kit customized development services.
SPR/BLI affinity assay services
Surface plasmon resonance (SPR) and biolayer interferometry (BLI) are frequently employed to determine the extent of binding between macromolecules. SPR technology is considered to be the "gold standard" for molecular interactions in the antibody field. This method has already been included in Chinese, US, and Japanese Pharmacopoeias.
Sino Biological has created high-quality proteins for use across several research areas, including Fc receptor, immune checkpoint, and biotinylated proteins.
Sino Biological’s integrated molecular interaction analysis platform can also provide antibody screening, consistency evaluation, epitope mapping, activity testing, and quality control services.
Service highlights

- Affinity assay of Fc receptor protein and antibody
- Affinity assay of complement and antibody
- Antibody screening and characterization
- Small-molecule affinity determination
- Biomacromolecular interactions
- Consistency evaluation

Image Credit: Sino Biological Inc.
Apoptosis assay services
Apoptosis assays can help illuminate the anti-tumor mechanisms of antibody drugs and offer a reference point for in vitro drug efficacy assessment.
Sino Biological's apoptosis assay service incorporates two primary methods: Annexin V/7-AAD assay and mitochondrial membrane potential assay.
Flow cytometry is leveraged for the detection of different types of apoptosis across a range of time periods to analyze the proportion of apoptotic cells, among other things.
Apoptosis detection methods and case studies
Annexin V/7-AAD
Assay Annexin V can be used as a sensitive probe for the detection of the PS exposed on the cell surface as it is a calcium-dependent phospholipid binding protein, with a high-binding capacity to PS.
7-AAD is a nucleic acid dye that does not have the capacity to penetrate cell membranes, but 7-AAD can permeate the cell membranes of cells in the mid- to late-stages of apoptosis, or even infiltrate dead cells and stain the nucleus red.
Using Annexin V and 7-AAD in combination can help identify early apoptosis and distinguish it from late apoptosis.
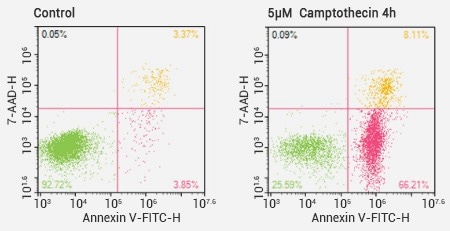
Flow cytometer analysis results of Annexin V/7-AAD double staining. Image Credit: Sino Biological Inc.
Mitochondrial membrane potential assay
The decrease in mitochondrial membrane potential is a significant event in the early stages of apoptosis. There are a variety of dyes, such as JC-1, Mito Tracker® Red, and rhodamine 123, that can be used to detect this change.
In healthy cells, JC1 aggregates to exhibit a red fluorescence. In apoptotic cells, JC-1 remains in monomeric form as it cannot accumulate within the mitochondria and instead shows green fluorescence.
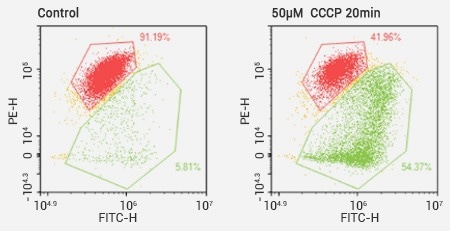
The analysis of mitochondrial membrane potential through JC-1 assays. Image Credit: Sino Biological Inc.
Cell proliferation inhibition assay service
Cell proliferation inhibition assays are vital for the determination of the biological activities of antibody drugs. Sino Biological can supply cell proliferation inhibition assay services along with a wide range of high-grade cytokine products.
Bioactivity assay methods validated by various hot targets
| EGFR |
HER2 |
HGFR |
VEGF |
VEGFR2 |
| FGFR |
CSF1R |
IGF1R |
CD123 |
IL2 |
| IL3 |
IL4 |
IL5 |
IL6 |
IL7 |
| IL9 |
IL10 |
IL11 |
IL13 |
IL15 |
| IL19 |
TPO |
FGF4 |
HGF |
NGF |
| EGF |
G-CSF |
GM-CSF |
FLT3 |
More Hot Targets |
Cytokine assay services
ELISA assays are the most frequently used techniques to quantify cytokines, which are used to assess the biological activity of antibodies by measuring the cytokines of target cells after co-culture with antibodies and other stimulating factors.
Sino Biological provides a range of cytokine assay services for different applications. Sino Biological has developed high-sensitivity, high-specificity cytokine ELISA detection kits for use in cytokine assays.
Applications
Immune checkpoint
The antagonist/agonist influence of immune checkpoints identifies the T cells to secrete cytokines such as IL-2.
Cytokine-targeting drugs
Anti-cytokine or receptor antibodies can impede the binding of the cytokines to their respective receptors and thereby influence the secretion of cytokines by cells. For instance, IL17 can induce IL-6 secretion by NIH/3T3 or HFF cells.
In vitro cytokine release assay
The cytokine release assay (CRA) is an in vitro assessment method which uses immune cells taken from healthy subjects.
Service workflow
- Confirmation testing program
- Contract confirmation
- Sending experimental materials
- Experimental testing
- Analyzed and reported test results
MLR assay service
Mixed lymphocyte response assay service
Mixed lymphocyte reaction (MLR) is an key in vitro model for the effective evaluation of lymphocyte activation, inhibition, or modification of function. To put it simply, various types of leukocytes are integrated and tested.
Three commonplace MLR assays include DC+T, PBMC+PBMC, and T+T. Among them, DC+T is a classic assay frequently used to assess the enhancement effect of antibody drugs on T-cell response.
For the purpose of the study, researchers must clearly determine the cell type for the MLR assay predicated on the action mechanism of the drug candidates.
The MLR assay can help identify the key differences in major histocompatibility complex (MHC) antigens between individuals. Particularly, this assay can determine the possible immune-activating or obstructive effects of a drug.
Sino Biological provides tailored antibody function assay services.
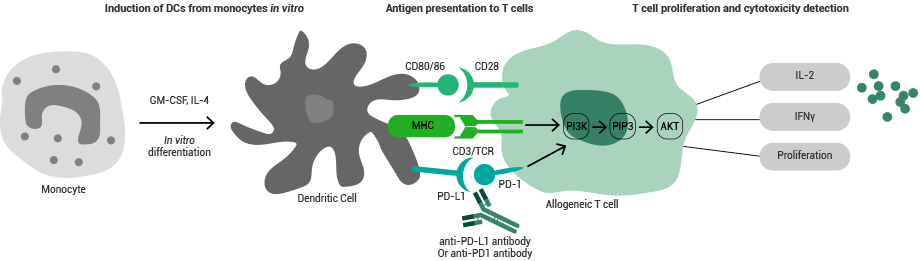
Principle of the MLR assay. Image Credit: Sino Biological Inc.
Service workflow
- Project discussion
- Contract confirmation and project initiation
- Shipping the testing samples
- Performing the assay
- Analyzing the results and issuing the assay report
Recommended products and services
Target proteins
Target proteins are vital reagents for the development of antibody drugs. As the world's main supplier of target proteins, Sino Biological can offer high-grade target proteins that encompass various elements of drug development including cell therapies, immune checkpoints, cytokines, enzymes, and receptors.
Human PD-1 protein (cat#: 10377-H02H)
High purity
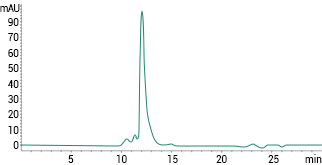
SEC-HPLC>90%. Image Credit: Sino Biological Inc.
ELISA binding activity validated
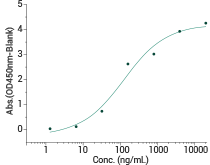
Immobilized Human PD-1 Protein (ECD, hFc Tag) at 2 μg/mL (100 μL/well) can bind Human PD-L2 Protein (ECD, His Tag) (Cat#: 10292-H08H), the EC50 is 45-280 ng/mL. Image Credit: Sino Biological Inc.
Antibody drug binding validated (BLI)
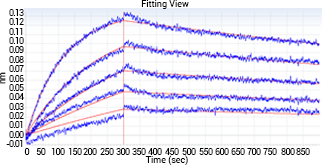
Using the Octet RED System, the affinity constant of Human PD-1 Protein (Fc Tag), bound to Atezolizumab was 0.3 nM. Image Credit: Sino Biological Inc.
Most-popular target proteins at a glance
| Her2 |
PD-1 |
EGFR |
CD47 |
TFRC |
| TIGIT |
CD38 |
TNFR-2 |
CD25 |
LAG3 |
| CD155/PVR |
TREM-2 |
CD122 |
B7-H3 |
CD40 |
| B7-H4 |
IL-18Rα |
CD28 |
PD-L1 |
DLL4 |
| CD19 |
CD16a |
FCGRT & B2M |
c-MET |
CD3D & CD3E |
| 4-1BB/CD137 |
PSMA |
CD3 epsilon/CD3e |
TROP2 |
CD32A |
| HER3/ERBB3 |
CTLA-4 |
BCMA |
CEACAM5 |
SIRP alpha |
| CD64 |
ILT4 |
FAP |
IL1R1 |
CD32B/Fcgr2b |
| IL-6R |
LILRB1 |
IL-3R alpha/CD123 |
ROR1 |
ILT3 |
| IL-18R beta / IL-18RAP |
CD22 |
CD39 |
HMGB1 |
IGF1R |
Control antibody production service
Sino Biological offers research-grade biosimilar control antibody production services of the highest standard. Furthermore, Sino Biological can provide high-purity and low-endotoxin control antibodies as positive controls for in vitro drug efficacy evaluation studies.
Service features
- Activity was validated by ELISA-binding activity and cell activity assays
- Successful production of 10,000+ antibodies
- High-quality delivery: low endotoxin (<1 EU/mg), high purity (SDS-PAGE & SEC-HPLC >95%)
- High-throughput and large-scale production capacities, ranging from μg to g
Service details
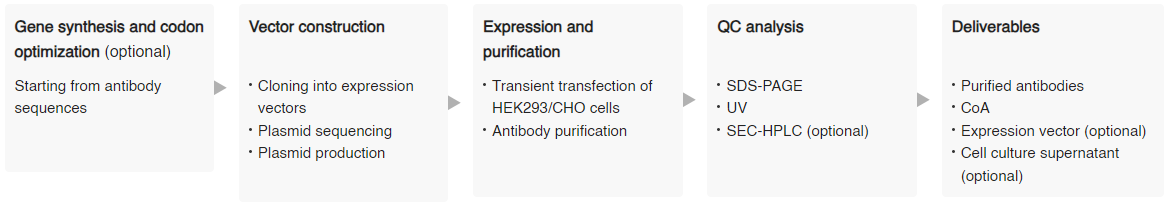
Note: Additional QC options are available upon request, including SEC-HPLC, endotoxin, affinity, ELISA, FACS, and more.
Featured case study
Anti-PDL1 antibody
High purity
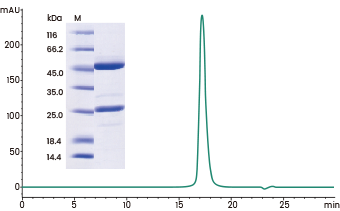
SDS-PAGE purity >96% and SEC-HPLC purity >98.77%. Image Credit: Sino Biological Inc.
Binding activity validated
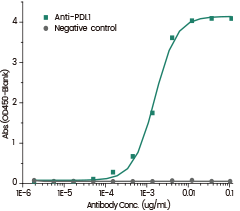
Anti-PDL1 Antibody binds the Human PDL1 Protein (His Tag), IC50=1.56 ng/mL. Image Credit: Sino Biological Inc.
Neutralization activity validated
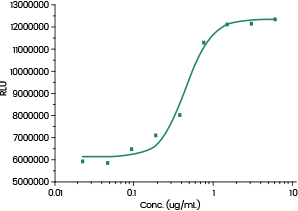
Measured by its ability to induce PD1/PDL1 pathway activation in a Luciferase receptor Assay System. Image Credit: Sino Biological Inc.
Cytokines
Cytokines are used extensively in experiments including cell proliferation inhibition assays, CRAs, and MLRs. Sino Biological has designed a range of cytokines (e.g., M-CSF, VEGF165, IL-15, and IL-21) with high purity, activity, and excellent batch-to-batch consistency to facilitate the ongoing development of antibody drugs.
Product advantages
- High purity
- HPLC verified
- Activity validated
- High lot-to-lot consistency
Human M-CSF/CSF1 (cat#: 11792-HNAH)
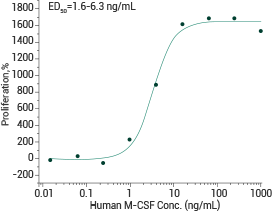
Measured in a cell proliferation assay using M‑NFS‑60 mouse myelogenous leukemia lymphoblast cells. Image Credit: Sino Biological Inc.
Human/Cynomolgus VEGF165 (cat#: 11066-HNAH)
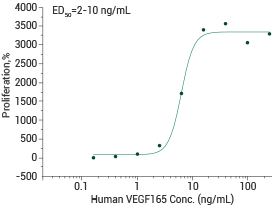
Measured in a cell proliferation assay using human umbilical vein endothelial cells (HUVEC). Image Credit: Sino Biological Inc.
ELISA kits
In vitro CRA is typically considered to be part of the preclinical safety assessment of therapeutic antibodies. The cytokines most frequently evaluated include IL-2, IL-6, IL-10, IFN-γ, and TNF-α.
Sino Biological offers cytokine ELISA kits of the highest standard, each of which has been tested in line with eight QC standards to ensure excellent performance and high batch-to-batch consistency. These kits can be used across various application scenarios of antibody drug development, including cytokine release and MLR assays.
Human IL2 ELISA kit (cat#: KIT11848)
Standard curve
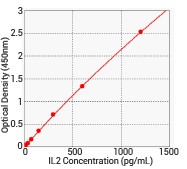
Standard Curve of Human IL-2 ELISA Kit. Image Credit: Sino Biological Inc.
Sensitivity
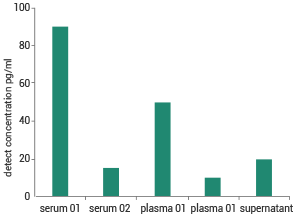
Detection of human serum, plasma and cell supernatant samples using Human IL-2 ELISA Kit. Image Credit: Sino Biological Inc.
Precision
Source: Sino Biological Inc.
| |
Intra-assay Precision |
Inter-assay Precision |
| Sample |
1 |
2 |
3 |
1 |
2 |
3 |
| N |
20 |
20 |
20 |
3 |
3 |
3 |
| Mean (pg/mL) |
50 |
128 |
769 |
54 |
136 |
779 |
| SD |
1.55 |
2.15 |
19.68 |
2.22 |
4.75 |
13.46 |
| CV(%) |
3.1 |
1.7 |
2.6 |
4.1 |
3.5 |
1.7 |
It has been verified that the intra/inter-precision of human IL-2 ELISA kit is less than 10%.
FACS antibodies
Sino Biological can provide a range of high-grade FACS antibodies (e.g., CD3, CD4, CD8, and CD86). Due to the high specificity, affinity, and exceptional signal-to-noise ratio, they can be employed in MLR assays to determine the levels of various T lymphocyte subpopulations or DC cells.
CD3D & CD3E antibody (PerCP), rabbit MAb (cat#: CT026-R301-C)
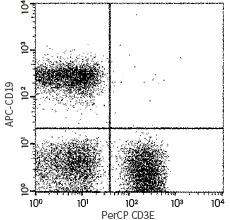
Flow cytometric analysis of Human CD3 expression on human peripheral blood lymphocytes. Image Credit: Sino Biological Inc.
CD4 antibody (APC), Mouse MAb (Cat#: 10400-MM08-A)
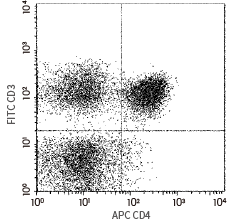
Flow cytometric analysis of Human CD4 expression on human peripheral blood lymphocytes. Image Credit: Sino Biological Inc.
CD86 antibody (FITC), Mouse MAb (Cat#: 10699-MM06-F)
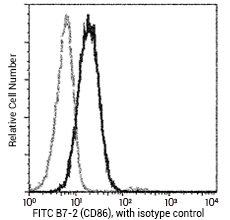
Profile of anti-B7-2 (CD86) reactivity on Daudi cells analyzed by flow cytometry. Image Credit: Sino Biological Inc.
FcyRs/FcRn proteins
Antibody drug efficacy is not only dependent on how well the Fab region binds to the target antigen but also on the interaction between Fc region–Fc receptor.
This interaction significantly impacts the half-life of antibody drugs and controls the antibody-dependent ADCC, ADCP, and CDC effects. In vitro identification of Fc–Fc receptor binding can be leveraged to forecast the functional potency of antibody drugs.
Presently, FcRn and FcγRs are the principal receptors for Fc–Fc receptor binding assays. Sino Biological has designed several recombinant FcγRs/FcRn products that encompass various isoforms and their mutants; these products can be used across a variety of applications including antibody screening, consistency evaluation safety validation, and when running bioactivity assays during the CMC stage.
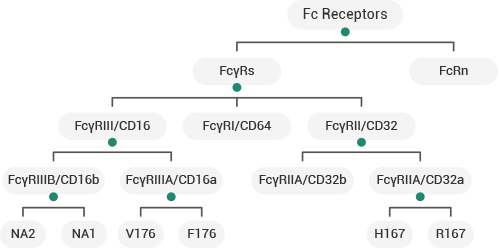
Human FcγRs/FcRn Products. Image Credit: Sino Biological Inc.
FcγRs/FcRn product features
- Assured batch-to-batch stability
- Bioactivity was validated by ELISA/SPR/BLI
- Expressed in HEK293/CHO cells, closer to the native structure
- High purity: SDS-PAGE ≥ 95% and SEC-HPLC ≥ 90%
- Multiple species to support various cross-species experiments
- Providing site-specific biotinylated proteins (Avi-tag)
Human FcγRIIIA / CD16a (F176) protein (cat#: 10389-H08H)
High purity
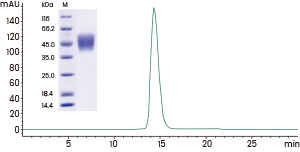
SDS-PAGE & SEC-HPLC >95%. Image Credit: Sino Biological Inc.
Antibody drug binding validated (BLI)
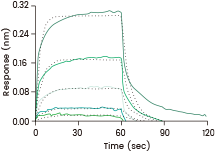
Loaded Human FcγRIIIA / CD16a (F176) recombinant protein (Cat. 10389-H08H) on His1K Biosensor, can bind Bevacizumab (IgG1) with an affinity constant of 1.9 μM as determined in a BLI assay (ForteBio Octet Red384). Image Credit: Sino Biological Inc.
SPR validated
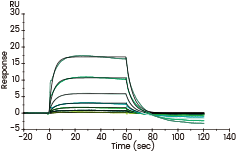
Captured Human FcγRIIIA / CD16a (F176) recombinant protein (Cat. 10389-H08H) on Anti-His Chip can bind Human IgG Fc (Cat. 10702-HNAC) with an affinity constant of 7.1 μM as determined in an SPR assay (Biacore T200). Image Credit: Sino Biological Inc.
About Sino Biological Inc.
Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.