Recombinant antibodies (rAbs), also called genetically engineered antibodies, are produced by cloning the antibody which is made of light and heavy chain DNA sequences.
In comparison to monoclonal antibodies that are produced with the help of traditional hybridoma techniques, rAbs provide benefits, like animal-free manufacturing, high lot-to-lot consistency, continuous supply and engineering advancement possibilities.
Provided the significance, recombinant antibodies are becoming essential tools that find application in basic research, diagnostics and clinical applications.
Antibody research and clinical development were reformed by the breakthrough of hybridoma technology in 1975. But for therapeutic purposes, the potency of murine-derived antibodies has been limited by human anti-mouse antibody responses, in which the murine antibodies are determined as foreign molecules by the human immune system.
In 1984, the first chimeric antibody was also identified as the first version of recombinant antibodies and was built by genetic engineering to decrease the immunogenicity of murine antibodies present in humans.
Almost 30% to 35% of the molecules have been derived from mouse antibody sequences, and 65% to 70% are from human antibody sequences. The consequent chimeric antibodies maintained the antigen-binding potential of the parental mouse antibodies.
The first step in developing therapeutic humanized antibodies is antibody chimerization. With the help of computer-aided molecular modeling and complementarity-determining region grafting technology, Sino Biological offers high-quality monoclonal antibody humanization services allowing a high degree of successful humanization (>90%).
Every full-length immunoglobulin (IgG) molecule consists of two light and heavy chains connected by disulfide bonds (Figure 1). Antibody fragments, like scFv, Fab and VHH, are small in size, thereby offering improved penetration of tissues or tumors compared to their full-length counterparts.
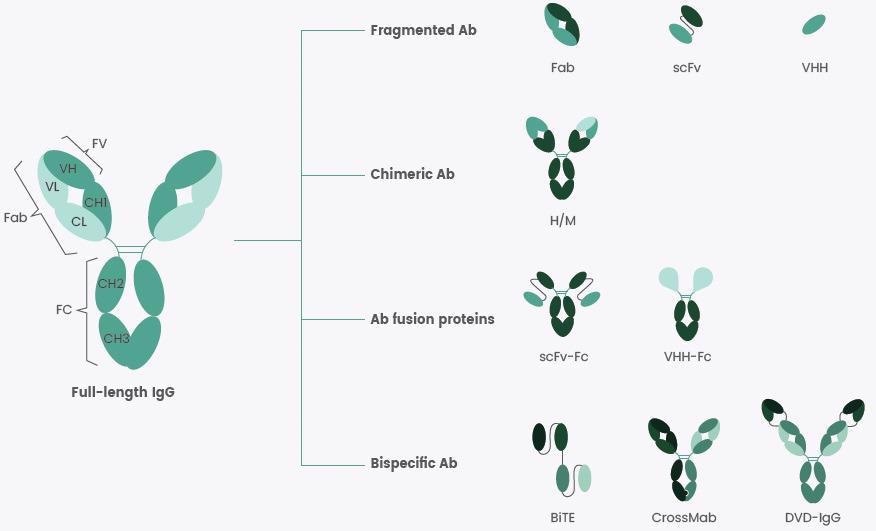
Figure 1. Some Recombinant Antibody Formats at a Glance. Image Credit: Sino Biological Inc.
This provides them with a hopeful future in immunotherapy, particularly in solid tumors. Moreover, they also have a brief half-life, which is considered useful as radioactive imaging agents.
But as there are not enough Fc regions, they cannot evoke Fc-mediated antibody effector functions, like complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC).
Initially, enzymatic digestion was utilized to fragment IgG antibodies. Pepsin splits the IgG heavy chains into the pivot regions following the disulfide bonds, thereby making a bivalent Fab fragment called F(ab’)2. Further, the fragment could be cleaved into two identical Fab fragments with the help of papain.
But this enzymatic cleavage technique is limited by the kinds of antibody fragments that have been produced. Moreover, it is inappropriate for industrial antibody production and purification. As a result of the progress in antibody engineering techniques, these issues can be resolved by generating antibody fragments recombinantly.
Following successful cloning and sequencing of the antibody genes, antibody fragments could be expressed in microbial expression systems, like mammalian systems (HEK293 cells), via transient transfection.
With the help of rich experience and expertise in recombinant production, Sino Biological has come up with a high-throughput (HTP) VHH expression platform (Figure 2) providing several VHH antibody production projects, with an overall success rate of over 90%.
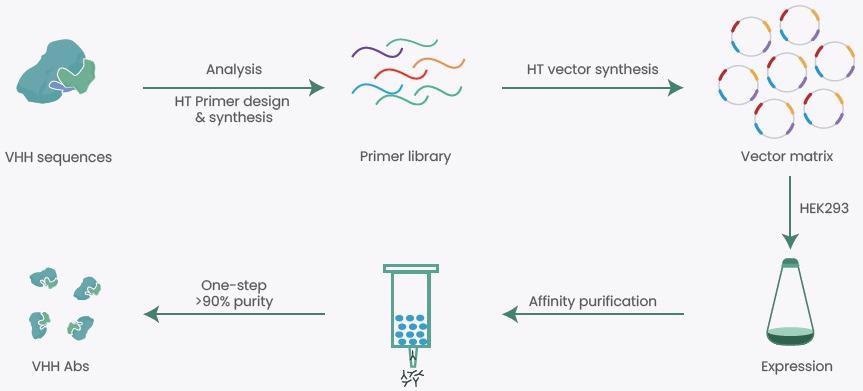
Figure 2. High-throughput (HTP) VHH Expression Platform. Image Credit: Sino Biological Inc.
Besides common VHH formats, dual- and multi-targeting VHHs can be expressed (Figure 3). Moreover, Sino can also express several other fragments with high affinity and specificity, like Fab and scFv.
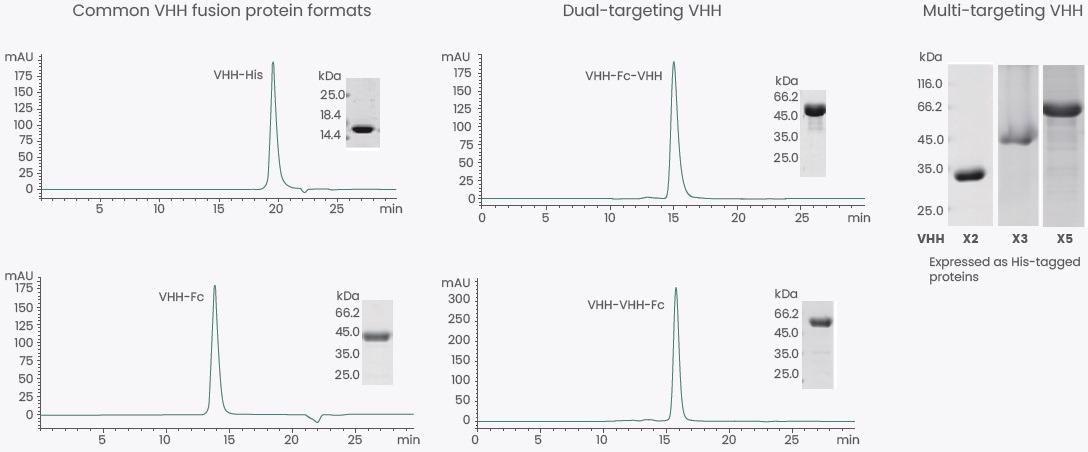
Figure 3. Expression of Various VHH Formats. Image Credit: Sino Biological Inc.
Dissimilar to conventional monoclonal antibodies, bispecific antibodies (bsAbs) are considered those with two binding sites that have the potential to identify two various epitopes or antigens on the same antigen. As a result of this special feature, bispecific antibodies have gained much attention from scientists and the drug industry.
As of this date, four bsAb drugs have been approved by the Food and Drug Administration (FDA), and more than 160 bsAbs are at present undergoing clinical trials for diabetes, cancer, Alzheimer’s disease and other diseases.
In the beginning, bispecific antibodies were produced by quadroma technology, but it is considerably challenging to downstream antibody manufacturing and purification. After the development of recombinant DNA technology in the last two decades, various bispecific antibody formats have appeared to adapt to the preferred target–product profile.
For the heavy chain mismatching issue to be resolved, Genentech initially suggested the “knob-into-hole” (KiH) technology, involving engineering CH3 domains to make either a “knob” or a “hole” in every heavy chain to induce heterodimerization.
In the same way, other technologies, like CrossMab and common light chain, are applied to handle the light chain mispairing issue.
In the mammalian cells, expressing bispecific antibodies are predominantly produced. As a result of several structural similarities between bispecific and monoclonal antibodies, several established purification processes for traditional mAbs are compatible with bispecifics.
Fc-fusion proteins
Fc-fusion proteins (also called Fc chimeric fusion proteins, Fc-Ig’s, and Fc-tag proteins) are homodimers comprising an IgG-Fc domain fused to a protein of interest, like a ligand, enzyme and peptide.
Even though monoclonal antibodies are present at the focal point of therapeutic biologics development, Fc-fusion proteins are also known to be a successful class of biopharmaceutical products, with a minimum of 13 drugs that have been approved by the European Medicines Agency (EMA) and FDA.
Besides therapeutic applications, Fc-fusion proteins act as detection reagents in fundamental research, such as immunohistochemistry, flow cytometry and protein binding assays. Indeed, linkage to the Fc domain has the ability to enhance the solubility and stability of a few binding collaborators.
Provided the size and requirement for glycosylation (majority of them are glycoproteins), Fc-fusion proteins are primarily produced in mammalian expression systems.
Concluding remarks
In recent times, progress happening in antibody engineering technologies has significantly improved the generation of recombinant antibodies in several formats as therapeutic agents. Already, there are over 100 antibody-based drugs approved by the FDA, and several antibodies are in their late-stage clinical studies at present.
Furthermore, engineered recombinant antibodies can find their use in numerous research applications: immunohistochemistry (IHC), western blotting (WB), immunofluorescence (IF), surface plasmon resonance (SPR) and flow cytometry (FC).
Since molecular biological and immunological technologies are on the rise, recombinant antibodies will be extensively utilized in fundamental scientific research and disease prevention, diagnosis and treatment.
Hence, this offers strong assistance for scientific research and following a healthier future for humans.
References
- Frenzel, A. et al. (2013). Expression of recombinant antibodies. Frontiers in immunology, 4, 217.
- Basu, K., et al. (2019). Why recombinant antibodies—benefits and applications. Current opinion in biotechnology, 60, 153-158.
- Butler, M. (2004). Animal cell culture and technology. Taylor & Francis.
- Ma, H., et al. (2017). Recombinant antibody fragment production. Methods, 116, 23-33.
- Zhao, L., et al. (2019). Genetic Engineering Antibody: Principles and Application. In IOP Conference Series: Materials Science and Engineering (Vol. 612, No. 2, p. 022045). IOP Publishing.
- Little, M. (2009) Recombinant antibodies for immunotherapy. Cambridge University Press.
- Ma, J., et al. (2021). Bispecific antibodies: from research to clinical application. Frontiers in Immunology, 12, 1555.
- Czajkowsky, D. M., et al. (2012). Fc-fusion proteins: new developments and future perspectives. EMBO molecular medicine, 4(10), 1015-1028.
About Sino Biological Inc.

Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offers pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.