In the last few years, multispecific antibodies, such as bispecific, trispecific, and tetraspecific antibodies, have become the breakthrough stars in antibody therapeutics. Compared with conventional monospecific antibodies, multispecific antibodies are now particularly important for biotech and big pharmaceutical companies as they offer several advantages, such as better specificity and targeting capabilities, as well as enhanced therapeutic effects.
The FDA has approved a large number of bispecific antibodies worldwide, and over 100 multispecific antibodies are being carefully evaluated in clinical trials.
Sino Biological has a wide array of products and services available to its customers with complete multispecific antibody development solutions.
Sino’s products cover different phases of the development process, including antibody development and optimization, animal model assessment, druggability evaluation, and process development. When taking these solutions on board, Sino Biological’s solutions allow its customers to expedite their multispecific antibody development and clinical research.
Mechanism of action of multispecific antibody drugs
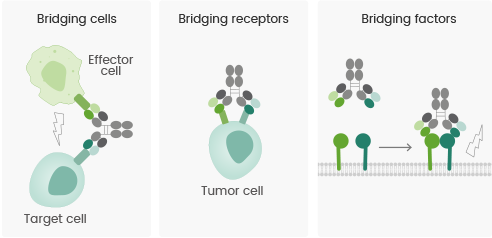
As reported by the “Bispecific Antibody Development Programs Guidance for Industry” published by the FDA in May 2021, the main action mechanism of bispecific/multispecific antibodies can be primarily divided into the following three categories:
- Bridging cells: The antibody connects immune cells with tumor cells. This bridging recruits and activates immune cells to kill tumor cells, which results in the redirection of cytotoxic effector cells.
- Bridging receptors: Several signaling pathways are constructed in the development of tumors; impeding a single signaling pathway cannot inhibit disease progression entirely. Bispecific antibodies can activate or inhibit several signaling pathways, creating coordinated effects.
- Bridging factors: The bivalent structure of bispecific antibodies intervenes in the formation of protein complexes and membrane receptor protein complexes. This increases the activity of antibody–drug conjugates or agonistic antibodies and generates a series of relative biological effects.
MOA of Multispecific Antibodies. Reference: doi: 10.1038/s41573-019-0028-1
Antibody discovery is where the development of multispecific antibody drugs begins, and the resulting outcomes are of utmost importance. Early drug candidates will feature in each phase of multispecific antibody drug development, and in turn, this process directly impacts the attributes of the drug molecules.
Sino Biological can offer four principal antibody development platforms and four primary protein expression platforms, which facilitate early development projects that evaluate multispecific antibody drugs to meet the various development needs of Sino’s scientific and industrial customers.
Four antibody development platforms
The structure of multispecific antibodies has an impact on their biological activity, making it a key area of their development. Sino Biological presents four principal antibody development platforms, including the Beacon® single B-cell platform, a phage-display platform, a FACS single B-cell sorting platform, and a hybridoma development platform.
These platforms take into account all processes, from antigen design and preparation to animal immunization, all of the way through to antibody production, which means Sino Biological can screen and determine antibody molecules that meet customer’s requirements.
Sino Biological offers phage display antibody library construction and screening services for several species, including mouse, rabbit, and chicken.
- Experience in supplying more than 5,000 rabbit mAbs;
- Successful screening rate of 90%
Our Beacon® platform automatically screens 10,000+ plasma cells, shortening the screening process from 1–2 months to one day.
- As fast as 35 days to obtain positive clones
- Screening more than 10,000 B cells simultaneously
The FACS B cell sorting platform offers high-throughput, effective, precise, and rapid manufacture of naïve paired light and heavy chain variable regions.
- All B cells can be processed within one day
- B-cell culture in 10–12 days
Sino Biological produces customized antigen design, animal immunization, clone screening, and antibody identification solutions.
- Project success rate > 90%
- Mouse mAb affinity can reach the pM level
Protein expression service
The likelihood and success of creating differential antibodies through screening is bolstered by the design of differential antigens. The selection of antigens and their quality are directly related to the successful production of antibodies with specificity and utility.
Drawing from 15 years of experience in protein research and development, Sino Biological can supply four main expression systems for mammalian cells, insect cells, E. coli cells, and HEK293/CHO stable cell lines. This allows the company to develop various types of antigens which ensures customers are provided with high-grade proteins.
Sino Biological enables high-throughput and high-yield expression of recombinant proteins and antibodies using CHO and HEK293 cells as hosts.
- Flexible culture volumes: 3 mL–1500 L
- 10,000+ proteins and antibodies expressed and purified successfully
With optimized expression vectors and virus packaging technology, Sino Biological provides high-quality recombinant expression and purification solutions.
- Recombinant protein purity > 95%;
- 1,000+ proteins successfully expressed and purified.
Sino Biological offers codon optimization to recombinant protein expression and purification.
- Rapid protein delivery within three weeks
- 1,000+ proteins successfully expressed and purified
Sino Biological is experienced in stable cell line development such as boosting cell productivity and improving cell status.
- Stable cell lines with antibody yields of up to 2–4 g/L;
- 15+ years of experience
Antibody optimization
Having acquired candidate antibodies from screening, they are subjected to modification and optimization processes to ensure the quality of the multispecific antibody drugs. Frequently used antibody optimization strategies include affinity maturation, antibody humanization, and stability improvement.
With vast experience in protein expression and antibody development and production, Sino Biological offers a complete service for antibody humanization, as well as AI-Powered affinity maturation, and multispecific antibody preparation. Therefore, customers can choose from a variety of optimized modifications and access high-grade antibodies to aid their research and development efforts.
Antibodies from hybridomas or murine ImmunoBanks are ill-suited for direct clinical use and can provoke immunogenic and toxic side effects in humans. To circumvent this issue, humanization modifications are necessary.
Leveraging computer-aided molecular modeling and complementarity-determining region grafting technology, Sino Biological can achieve a high degree of successful humanization through its efficient and reliable antibody humanization services (>95%).
- 100% success rate
- Affinity validated by ELISA/SPR/BLI
- Rapid delivery times: 3-4 weeks to deliver desired humanized antibodies
Sino Biological can supply antibody expression services in the microgram-to-gram range based on its mammalian cell expression platform. Utilizing efficient expression vectors, transfection reagents, an optimized proprietary medium, and high-density cell suspension culture technology, Sino Biological is able to meet the different application needs of its customers.
- 14 kinds of multi-specific antibody expression experience
- Access to several purification methods: Protein A/G/L, IEC, SEC
- High quality delivery: SEC-HPLC > 95%, endotoxin < 1 EU/mg
In vitro efficacy evaluations delivers data for the early screening of multispecific antibodies. Sino Biological has created an in vitro activity assay platform for antibody drugs. The platform has been developed to support the various multispecific antibody drug development projects its customers conduct and facilitates the efficient completion of in vitro efficacy assays.
- Comprehensive services and reagents, multi-dimensional efficacy evaluation system
- ISO 9001 & CNAS certified, rigorous quality management system
- With extensive experience, Sino Biological can supply an SPR/BLI affinity assay and an antibody-binding activity assay
Druggability assessment
Druggability is an important element of the molecule and is largely identified during the early stage of molecular screening. It is absolutely vital to make sure druggability is taken into account as it can have an impact on the cost of process development and the emerging risks throughout the clinical stage.
Therefore, druggability assessment is a key factor between drug discovery and drug development. To ensure customers are able to select the best candidates, Sino Biological has established a comprehensive in vitro efficacy evaluation platform.
Furthermore, Sino offers protein expression services and high-grade cytokines for cell proliferation inhibition assays and the mixed-lymphocyte reaction (MLR) assay service, assisting in the druggability evaluations of multispecific antibody drugs.
To assess the druggability of antibodies, it is essential to check their specific affinity aligns with their respective antigens. This demands the selection of the most suitable antigen for the antibody development project.
Sino Biological can supply a range of recombinant protein expression systems that have been fine-tuned to meet a variety of research needs, which makes the possibility of achieving successful outcomes in research projects more likely.
- Extensive QC testing: SDS-PAGE, SEC-HPLC, FACS, ELISA, cell assays, BLI/SPR, etc.
- Four expression systems: E. coli, mammalian, insect, and CHO/HEK293 stable cell line development
- HTP expression capabilities facilitate the rapid discovery of multispecific antibody drugs
Making sure drugs are both safe and demonstrate good efficacy is vital for the successful development of new pharmaceuticals. Thus, functional tests are a key element of druggability evaluation. Sino Biological offers SPR/BLI affinity assay and antibody-binding activity assay services and has set up an in vitro activity assay platform for antibody drugs. These assessments and services help establish the binding specificity and affinity of antibodies to their target proteins.
- Comprehensive services and reagents, multi-dimensional efficacy evaluation system
- ISO 9001 & CNAS certified, rigorous quality management systems
- Vast experience, well-established and reliable methodologies
High-quality cytokines and ELISA pair sets
Cytokines are used extensively throughout cell proliferation inhibition assays and MLR assays. Cell proliferation inhibition assays are vital when identifying the different biological activities of antibody drugs. Therefore, MLR assays are especially useful for the assessment of the effects test drugs have on human primary T-cell functions and for evaluating the antigen-presentation effect of test drugs in T-cell activation mediated by primary APCs.
Sino Biological can supply a wide range of cytokines with high activity, purity, and inter-batch consistency, including M-CSF, VEGF165, IL-3, and IL-4, which are used extensively in cell proliferation inhibition assays and MLR assays.
Sino Biological has developed a range of top-quality ELISA Pair Sets for IL-4, IL-6, IL-8, and IFN-gamma, which can be utilized when measuring the side effects of antibody drugs. For instance, showing how the binding of antibody drugs to lymphocyte Fc receptors provokes the release of ADCC and cytokines.
Animal model evaluation
It is of primary importance to leverage the appropriate animal models for the analysis of pharmacokinetics, efficacy, and safety to ensure the successful preclinical development of multispecific antibodies. However, a large number of candidate antibody drug molecules are required to ensure validation and animal experiments run as intended.
Sino Biological has years of experience and expertise in customized antibody production and can supply large-scale multispecific antibody production services for preclinical antibody drug production.
Sino Biological offers PK/anti-drug antibodies (ADA) antibody preparation services, serum concentration analysis, and immunogenicity assays for multispecific antibodies, supporting the analysis of candidate molecules and pharmacodynamic experimental design.
Sino Biological has set up a large-scale recombinant antibody expression platform that meets the demands of high-throughput and large-scale production of antibody drugs.
Sino Biological’s platform delivers recombinant antibody production services in the milligram to kilogram range. Its ISO-certified manufacturing facility is a certified environment which guarantees that all antibody production takes place within compliance guidelines, thus delivering high-grade products.
- 80+ bioreactors: simultaneously supporting dozens of antibody projects
- High-quality delivery: SEC-HPLC > 95%, endotoxin < 1 EU/mg.
- Scalable production capabilities: culture volumes vary from shake flasks to 1500 L bioreactors.
During the development of multispecific antibody drugs, anti-idiotype antibodies take up a central role in the detection and quantification of antibody-drug levels in animal or human serum, making them key detection reagents for PK studies.
Moreover, they act as a crucial reference standard for immunogenicity analysis. To accelerate drug development, Sino Biological offers an extensive range of services, such as antigen preparation, anti-idiotype antibody development, detection method establishment, and kit development.
- Five Antibody Development Platforms: hybridoma, Beacon®, FACS B cell, phage display, and pAb technologies
- High Quality: high titer, high specificity, and low cross-reactivity with human IgG
- Multiple Purification Methods: Protein A, antigen affinity, total human IgG, Isotype IgG
- Rich Experience: serving clients all over the world with a success rate >95%
Process development clinical studies
Sino Biological offers an extensive range of products and services, such as cell line development service, PK antibody development service, ADA antibody development service, and CHO cell culture media. The availability of such products supports the development process and clinical research of multispecific antibodies.
Multispecific antibody CMC development starts with the creation of efficient and stable cell lines, and it has a direct impact on the process complexity, production cost, and overall quality of the final product.
Beginning with the customer’s cDNA or antibody sequence, Sino Biological offers cell line development services which range from expression vector construction to screening for high-yield and stable cell lines.
- Fast delivery: as quick as eight weeks from gene to purified product
- High yield, with an expression level of no less than 2–4 g/L
- Officially licensed CHO-K1 cell lines to support IND, NDA, and BLA applications
- Variety of service packages on offer
PK antibody development service
PK studies are critical for the assessment of efficacy and evaluating the safety of multispecific drugs throughout the drug development process. For effective quantitative testing of drug levels in animal or human serum, Sino Biological can supply a range of anti-idiotype antibody generation service packages. These packages have been created to support preclinical and clinical PK studies of multispecific drugs.
- Four antibody development platforms: hybridoma, phage-display, Beacon® single B-cell, and FACS single B-cell platforms
- High quality: high specificity and sensitivity
- One-stop service from antigen preparation, antibody development, and ELISA kit generation through to analytical method development
- The success rate of over 95%, serving customers across the globe
ADA antibody development service
Anti-drug antibodies (ADAs) that the body produces may influence the pharmacokinetic and pharmacodynamic properties of multispecific drugs. Sino Biological has developed high-specificity, high-affinity, and high-sensitivity ADA antibodies for customers, providing assistance for the evaluation of immunogenicity of multispecific drugs in preclinical and clinical studies.
- High quality: high titer, high specificity, and low cross-reactivity with human IgG
- Multiple purification methods: Protein A, antigen affinity, total human IgG, Isotype IgG
- Vast experience: serving clients across the globe with success rate >95%
- Well-established rabbit pAb technology, offering fast and standard rabbit pAb production packages
Sino Biological has developed the SMM CHO-GSI medium (Cat#: MCHOGSI) and SMM CHOGS-C3 medium (Cat#: MCHOGS-C3), both of which are well-suited to the clone screening of GS-deficient CHO-K1 cells, as well as large-scale suspension cultures, and stable expression of recombinant proteins and antibodies. These media formulations are effective when it comes to supporting the development and production of multispecific drugs, offering efficient and reliable solutions for biopharmaceutical companies.
- Animal-component-free, serum-free, antibiotic-free media.
- Recommended for clone screening and suspension culture of GS-deficient CHO cells.
- Supporting high-cell-density growth and high expression of recombinant proteins.
- Stringent quality control and stable product batches.
Multispecific antibody webinar
Development of multi-specific therapeutic antibodies via computer aided design
Video Credit: Sino Biological Inc.
About Sino Biological Inc. 
Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.