Molecular test, or nucleic acid amplification, has been recommended by the FDA for the diagnosis of the COVID-19 virus. Nucleic acid amplification testing necessitates the collection of respiratory samples from patients, with nasopharyngeal swabs being the most commonly used technique.
Molecular tests may provide a false negative result, should the level of viral RNA in a particular sample be too low for detection. Additionally, results may be skewed if samples are not properly taken. According to clinical studies, the sensitivity of molecular tests is approximately 50%.1
New tools, such as serological testing, are becoming more readily available. It is important to gain enhanced understanding of various COVID-19 testing approaches, so that existing tools can be best used to successfully control the spread of SARS-CoV-2.
Immune systems naturally create antibodies to respond to infections. Serological tests are diagnostic approaches employed to identify antigens and antibodies in patient samples.
The serological test usually offers a number of advantages when compared to the molecular test. These include a low detection threshold, high throughput, convenient and stable sample collection, and low workload.
In order to characterize the disease’s pervasiveness and spread, serological testing is already widely used, with demand for SARS-CoV-2 serological testing quickly increasing.
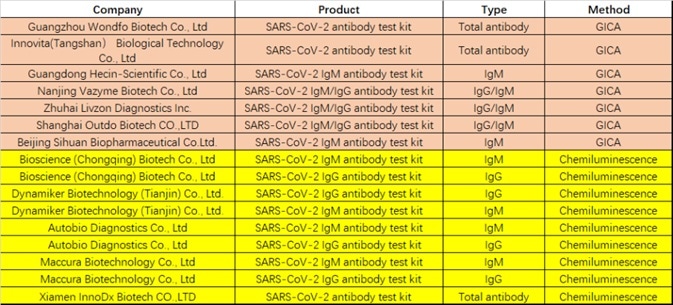
Twelve tests currently have emergency use authorization from the FDA (see Table 1), with sixteen serological tests currently approved by the CFDA for emergency use (see Table 2). These assay formats are comprised of chemiluminescence immunoassay, colloidal gold immunoassay, and ELISA.
Table 1. Emergency Use Authorizations for COVID-19 by FDA. Source: ACROBiosystems
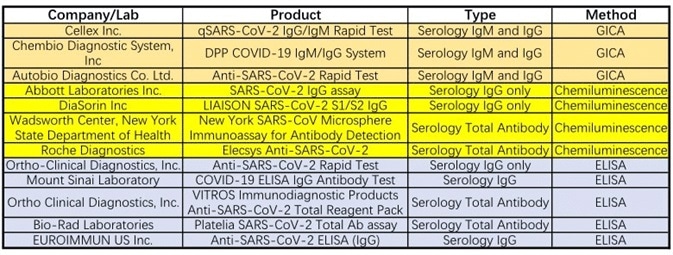
Table 2. COVID-19 diagnostic approved by CFDA. Source: ACROBiosystems
A summary of the mechanism and performance of each of the above three formats is included in Table 3, Figure 1, Figure 2 and Figure 3.
Table 3. Comparison of three assay formats. Source: ACROBiosystems
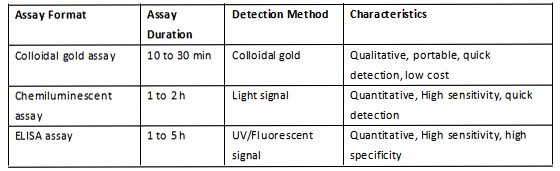
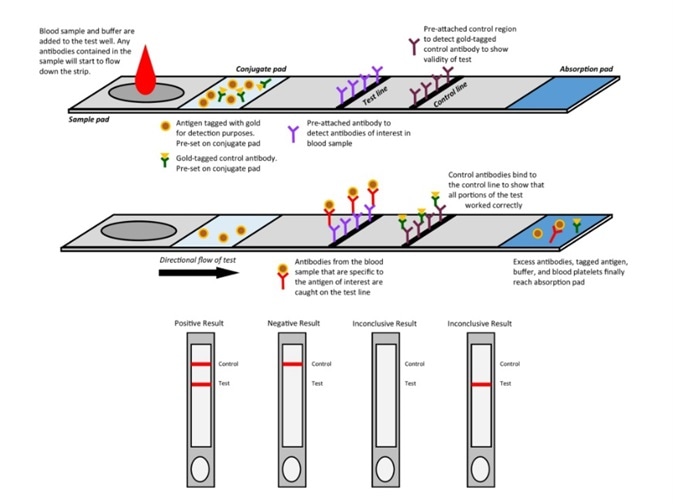
Figure 1. Mechanism of colloidal gold assay. Image Credit: ACROBiosystems
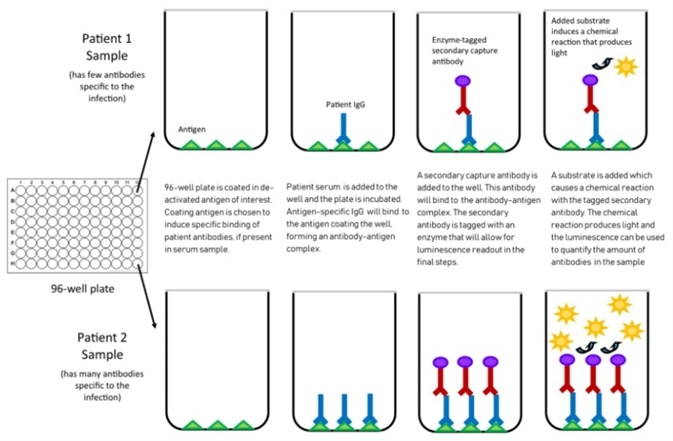
Figure 2. Mechanism of chemiluminescent assay. Image Credit: ACROBiosystems
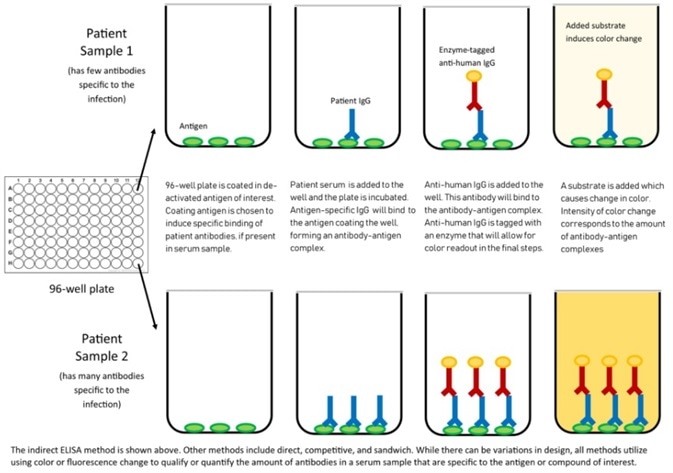
Figure 3. Mechanism of ELISA. Image Credit: Center for Health Security
All three of these methods utilize antigens to capture a target antibody, but, the test’s accuracy is considerably affected by the antigen’s reagents.
In order to better support development of serological test kits, ACROBiosystems has designed and optimized SARS-CoV-2 antigen products for serological test kits. These include S1, N and wild type RBD, alongside mutant RBD proteins with a variety of tags.
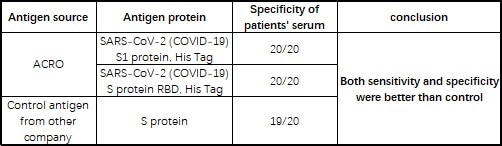
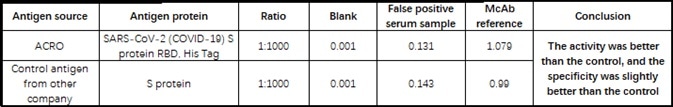
The company’s products have been verified by a wide range of customers using its serological assay platforms, with many providing positive feedbacks (see Table 4).
Recently, Babson Diagnostics has successfully launched its chemiluminescent COVID-19 antibody detection kit. The kit incorporates ACRO's biotin-labeled S1 protein, possessing a sensitivity of up to 93.2% and a specificity of 100%.2
Table 4. Feedback data using ACRO’s SARS-CoV-2 reagents. Source: ACROBiosystems
Application in GICA
Application in ELISA
ACROBiosystems has also created the Detection of Anti-SARS-CoV-2 antibody assay kit (using Spike protein RBD), which uses an indirect ELISA method. This approach coats SARS-CoV-2 S protein RBD, His Tag (Cat.No. SPD-C52H3) to find anti-SARS-CoV-2 IgG antibody in serum.
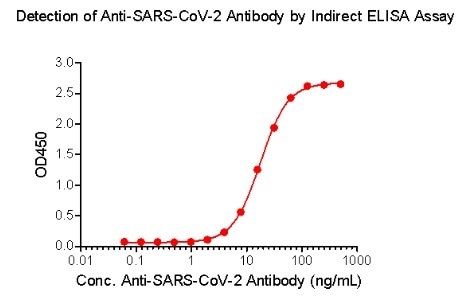
Figure 4. Indirect ELISA method to detect anti-SARS-CoV-2 IgG antibody. Image Credit: ACROBiosystems
ACRO’s verified protein reagents can be supplied in large quantities, and tailored to meet custom requirements.
The sensitivity of N protein binding to Anti-N mAb is 0.02 ng/mL as verified by ELISA
SARS-CoV-2 (COVID-19) Nucleocapsid protein, his tag
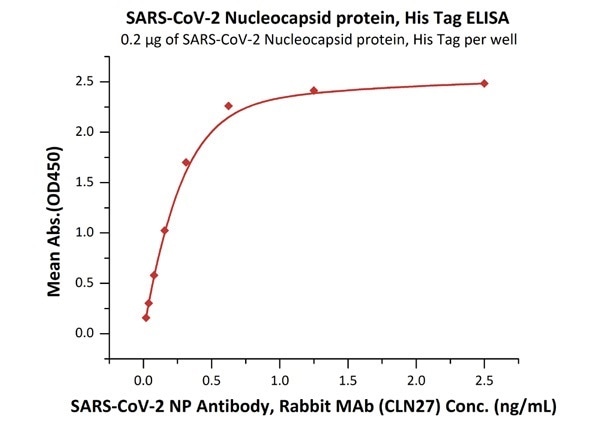
Figure 1. Immobilized SARS-CoV-2 Nucleocapsid protein, His Tag (Cat. No. NUN-C51H9) at 2 μg/mL (100 μL/well) can bind SARS-CoV-2 NP Antibody, Rabbit MAb (CLN27) with a linear range of 0.02-0.3 ng/mL. Image Credit: ACROBiosystems
The sensitivity of S1 protein binding to ACE2 protein is 0.2 ng/mL as verified by ELISA
SARS-CoV-2 (COVID-19) S1 protein, his tag (MALS verified)
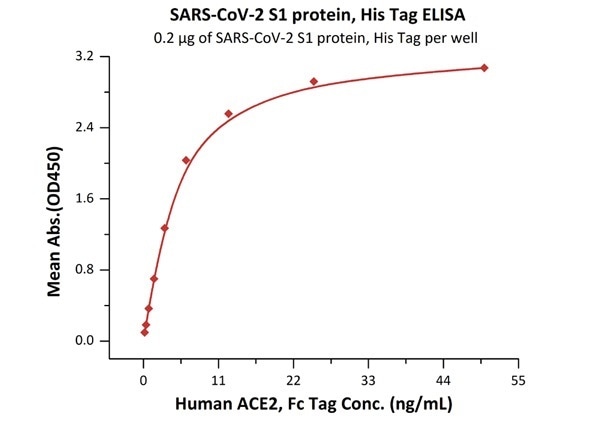
Figure 2. Immobilized SARS-CoV-2 S1 protein, His Tag (Cat. No. S1N-C52H4) at 2 μg/mL (100 μL/well) can bind Human ACE2, Fc Tag (Cat. No. AC2-H5257) with a linear range of 0.2-6 ng/mL. Image Credit: ACROBiosystems
The sensitivity of RBD protein binding to ACE2 protein is 8 ng/mL as verified by ELISA
SARS-CoV-2 (COVID-19) S protein RBD, his tag (MALS verified)
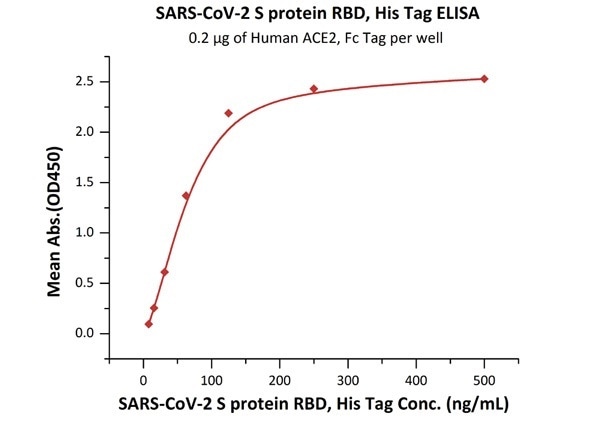
Figure 3. Immobilized Human ACE2, Fc Tag (Cat. No. AC2-H5257) at 2 μg/mL (100 μL/well) can bind SARS-CoV-2 S protein RBD, His Tag (Cat. No. SPD-C52H3) with a linear range of 8-125 ng/mL. Image Credit: ACROBiosystems
References
- Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019
- Babson Diagnostics Launches COVID-19 Serology Test for IgG Antibodies with 100% Specificity in Austin
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.