COVID-19 resurgence is putting health and lives at risk. The latest vaccine developments provide much-needed hope for the effective control and arrest of the pandemic. These developments stem from the unrelenting efforts of scientists from numerous labs and pharmaceutical companies, moving traditional and also newer technology-based vaccines through clinical pipelines at a record pace.
Pfizer/BioNTech released their Phase III clinical trial data on the 9th of November, revealing that their mRNA vaccine offers an effective rate of approximately 95% with no reports of serious side effects.
Pfizer/BioNTech was the first organization to release a positive Phase III trial result - a major milestone. Moderna issued a preliminary analysis of this 30,000-volunteer study on the 16th of November, reporting that the vaccine was found to be 94.5% effective at preventing COVID-19. This result was based on 95 cases of symptomatic infection, with 90 of these in a placebo group.
Vaccine effectiveness and safety remain the primary focus of any vaccine development and antibody-drug enhancement (ADE) risk was found to pose a safety concern that has to be addressed as part of the clinical evaluation.
ADE has the potential to increase severity in multiple viral infections and this has been documented as occurring via two specific mechanisms in viral infections. Firstly, ADE prompts an increase in viral infection and replication by enhanced antibody-mediated virus uptake into Fc gamma receptor IIa (FcγRIIa)-expressing phagocytic cells. Secondly, ADE triggers increased immunopathology and inflammation by immune complex formation or excessive antibody Fc-mediated effector functions.
ADE was initially described with Hawkes in 1964, who had witnessed this with the arbovirus. Scott Halstead first identified ADE in 1977, noting that the antibodies generated from a first dengue infection could occasionally worsen the symptoms from a second infection. ADE has since been seen in MERS, SARS, and a range of other human respiratory viruses (Figure 1).
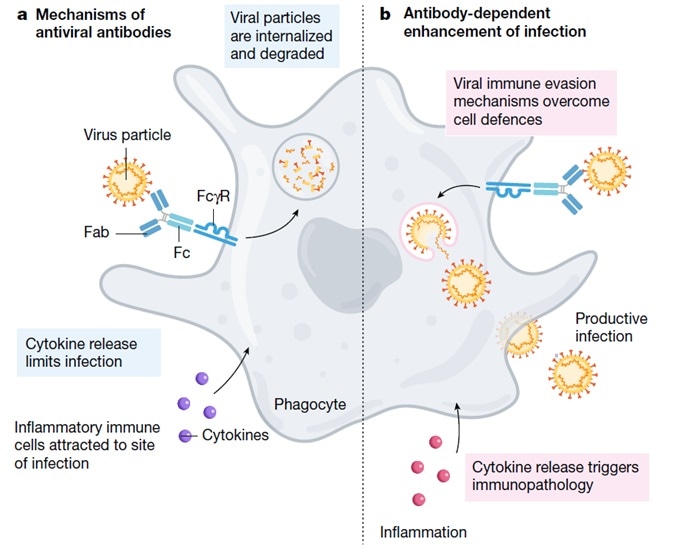
Figure 1. Mechanism of ADE. Image Credit: ACROBiosystems
The mechanism of ADE was not completely explained at this time, but with the knowledge of ADE’s existence, scientists were better equipped to determine ADE related antigen, modifying lead molecules as necessary in order to make these more effective and safer.
There have been no instances of ADE reported during the COVID-19 vaccine’s pre-clinical/clinical development, but further ADE related research should be undertaken in order to avoid potential side effects.
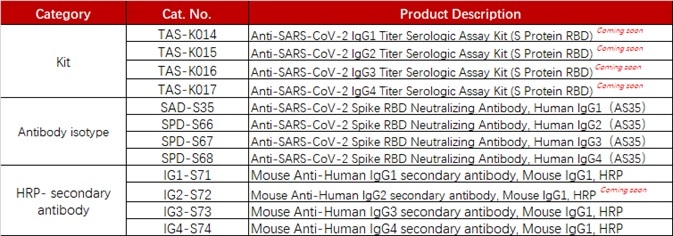
ACROBiosystems has established a range of products suitable for measuring anti-SARS-CoV-2 antibody isotypes including IgG1, IgG2, IgG3, and IgG4.
Product list
Source: ACROBiosystems
Find out more
ACROBiosystems has also developed various COVID recombinant proteins and kits to support vaccine development and evaluation. These include both Spike trimer proteins and antibody titer assay kits suitable for IgG/IgM, the antigen, and the neutralizing antibody.
Both the ACRO RBD/Spike antibody titer assay kit and neutralizing antibody titer assay kit are CE certified, having been specifically designed to support diagnostic and vaccine evaluation. These kits are robust, offering convenient, accurate, fast, high throughput with a high signal to noise ratio.
Find out more
Assay data
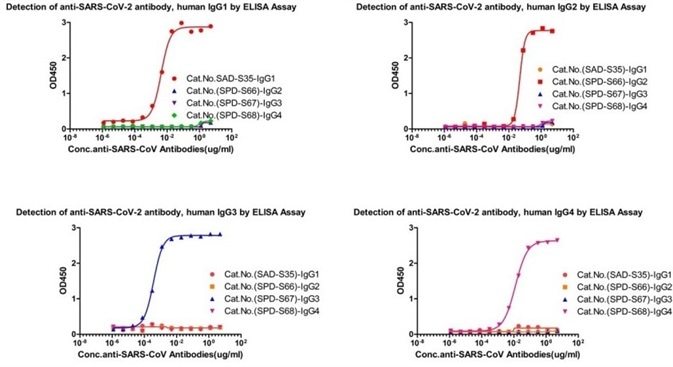
Figure 1. Cross-validation results of four IgG antibody subtypes detection. Image Credit: ACROBiosystems
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.