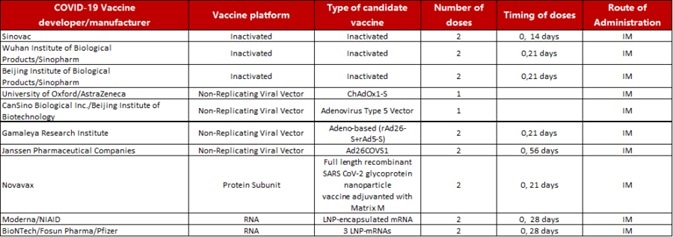
According to a plethora of documents prepared by the World Health Organization regarding COVID-19 candidate vaccines globally, 44 candidate vaccines are in clinical trials, 10 of which are at Phase III (Table 1). In addition, there are 154 candidate vaccines in the preclinical stage across the world.
Table 1. Ten candidate vaccines in Phase III clinical trial. Source: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
A standard development cycle for a vaccine is usually between 10 and 15 years. However, due to the scale of the COVID-19 public health emergency, researchers and scientists are working extremely hard to bring down the development cycle to just a year or two.
Due to the accelerated development of the vaccine and the incomplete understanding of the vaccine's immune responses, extra caution should be taken when assessing the efficacy and safety of the vaccine.
Two significant studies of the COVID-19 vaccine from Johnson & Johnson and AstraZeneca previously suspended trials because of potential safety concerns. However, both companies announced last Friday that trials are set to recommence.
According to the FDA presenting strict standards in their June 2020 guidance document, ‘Development and Licensure of Vaccines to Prevent COVID-19’, clinical trials are being conducted in the appropriate manner.
If a vaccine appears to demonstrate the required safety and effectiveness standards, approval for use or authorized for emergency use will be granted. Furthermore, the continuation of vaccine safety monitoring post-approval and authorization can reveal other adverse events that may have been missed during clinical trials.
Researchers will quickly investigate any unexpected adverse events to assess whether it is a legitimate safety concern. Researchers will then make a decision as to whether any changes are required in U.S. vaccine recommendations. This level of monitoring is vital to ensure that the advantages continue to outweigh the risks for people who receive vaccines.[1]
The road is still long as far as COVID-19 vaccine development and testing are concerned. With their expertise in specialized recombinant protein, ACROBiosytems has developed a series of COVID-19-related protein products, including S trimer protein and the titer measurement kits to assist vaccine development.
Application 1: Vaccine immunogenicity evaluation ----- IgG antibody measurement
Recommended product 1: SARS-CoV-2 S protein, his tag, super stable trimer (MALS & NS-EM verified)
Catalog number: SPN-C52H9
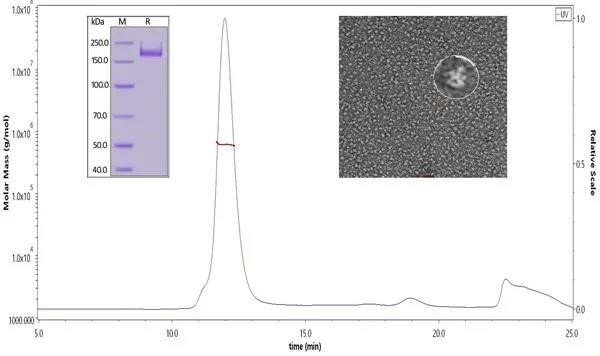
Figure 1. ACRO SARS-CoV-2 S protein was verified through SDS-PAGE, SEC-MALS, and negative staining electron microscopy. Our S trimer protein has over 90% purity with an MW of 550-660 kDa. According to the data of negative staining electron microscopy, the S trimer protein has a similar size and appearance compared to references. Image Credit: ACROBiosystems
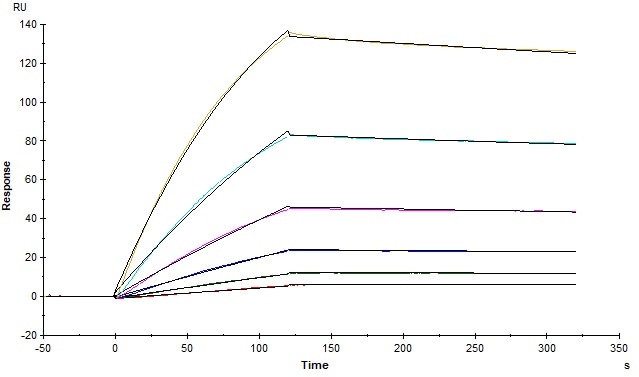
Figure 2. The binding affinity of SARS-CoV-2 S trimer (Cat. No. SPN-C52H9) and human ACE2 (Cat. No. AC2-H5257) was evaluated using Biacore. The KD was 5.82 nM. Image Credit: ACROBiosystems
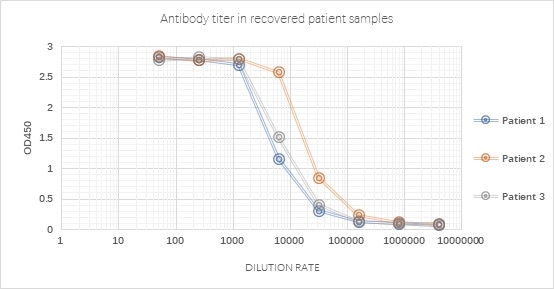
Figure 3. Antibody measurement in recovered patient samples using SARS-CoV-2 S trimer (Cat. No. SPN-C52H9) with a very good signal-noise ratio. Image Credit: ACROBiosystems
Recommended product 2: Anti-SARS-CoV-2 antibody IgG titer serologic assay kit (S protein trimer)
Catalog number: TAS-K007
A high-speed, effective assay kit for IgG antibody quantification in serum/plasma samples. This already well-known technique has excellent beneficial qualities, such as low background and a good signal to noise ratio.
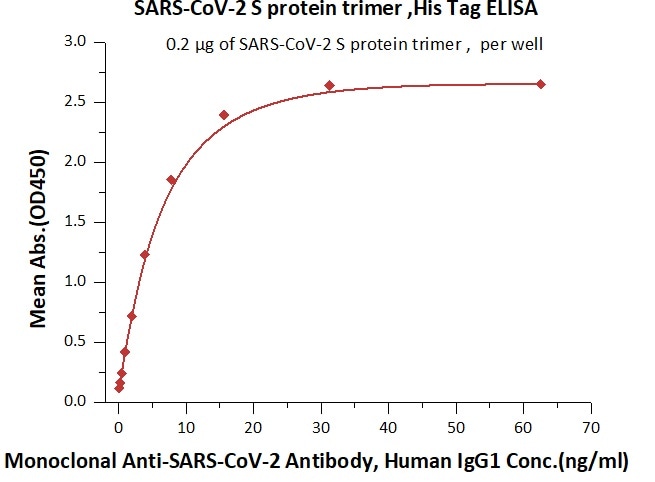
Figure 4. Immobilized SARS-CoV-2 S protein at 2 μg/mL (100 μL/well) can bind Monoclonal Anti-SARS-CoV-2 Antibody, Human IgG1 in 1:50 human serum. Detection was performed using the HRP-Conjugated Anti-human IgG antibody with a sensitivity of 12 ng/mL. Image Credit: ACROBiosystems
Recommended product 3: Anti-SARS-CoV-2 antibody IgG titer serologic assay kit (Spike protein RBD)
Catalog number: TAS-K002
An effective assay kit for rapid IgG antibody quantification in serum/plasma samples. This traditional method also has its benefits, including low background and a good signal to noise ratio.
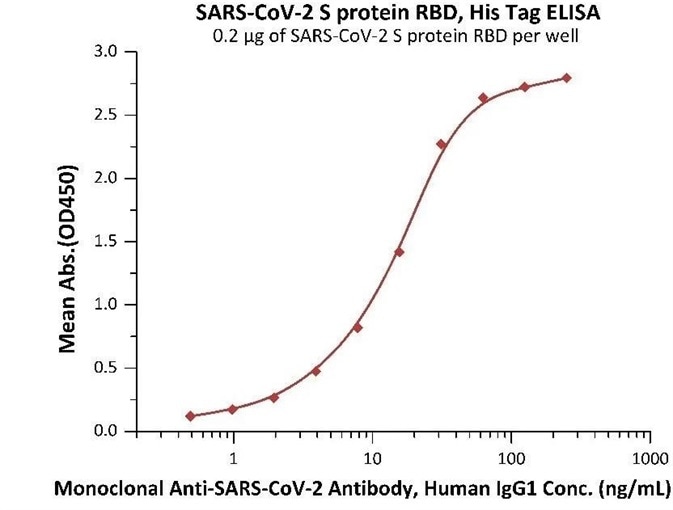
Figure 5. Immobilized SARS-CoV-2 S protein RBD at 2 μg/mL (100 μL/well) can bind Monoclonal Anti-SARS-CoV-2 Antibody, Human IgG1 in 1:50 human serum. Detection was performed using the HRP-Conjugated Anti-human IgG antibody with a sensitivity of 24 ng/mL. Image Credit: ACROBiosystems
Application 2: Vaccine clinical immunogenicity evaluation ----- Neutralizing antibody measurement
Recommended product 1: Anti-SARS-CoV-2 neutralizing antibody titer serologic assay kit
Catalog number: TAS-K003
This kit has an intended use for serologic measurement for the titer of Anti-SARS-CoV-2 neutralizing antibody throughout serum/plasma samples.
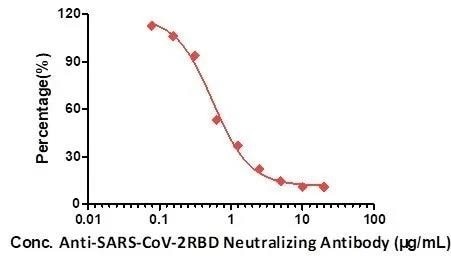
Figure 6. A neutralizing titer of Anti-SARS-CoV-2 Neutralizing Antibody, Human IgG1 (Cat.No. SAD-S35) measured by Anti-SARS-CoV-2 neutralizing antibody titer serologic assay kit (Cat. No. TAS-K003). Image Credit: ACROBiosystems
Recommended product 2: Anti-SARS-CoV-2 RBD potent neutralizing antibody, chimeric mAb, human IgG1 (AM128)
Catalog number: SPD-M128
This neutralizing antibody was authenticated via the utilization of a pseudotyped virus. It is developed as a convenient reference for the vaccine evaluation assays.
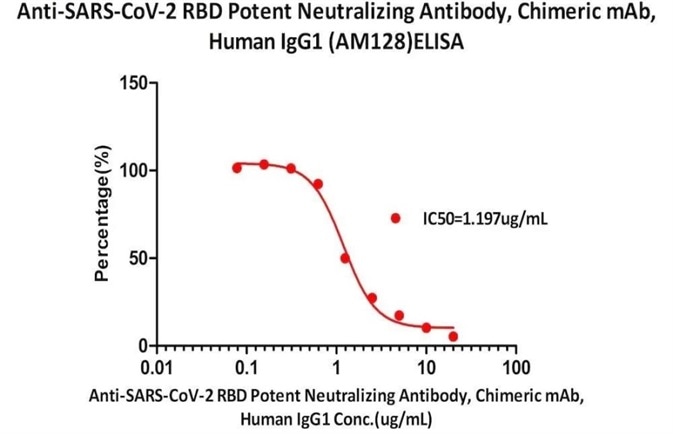
Figure 7. Serial dilutions of Anti-SARS-CoV-2 RBD Potent Neutralizing Antibody, Chimeric mAb, Human IgG1 (AM128) (Cat. No. SPD-M128) was detected by SARS-CoV-2 Inhibitor screening Kit (Cat. No. EP-105) with a half maximal inhibitory concentration (IC50) of 1.197 μg/mL. Image Credit: ACROBiosystems
Application 3: Vaccine post-market evaluation ----- Neutralizing antibody measurement
Recommended products
Table 2. Source: ACROBiosystems
| Catalog number |
Products |
Application |
| SPD-C52H1 |
SARS-CoV-2 (COVID-19) S protein RBD, His Tag |
Lateral Flow Assay |
| AC2-H52H8 |
Human ACE2 /ACEH Protein, His Tag (MALS verified) |
Furthermore, ACRO developed an antibody isotyping kit, which can be utilized for further anti-SARS-CoV-2 IgG1, IgG2, IgG3, and IgG4 testing. Additional validated kits will become available for future COVID-19 studies.
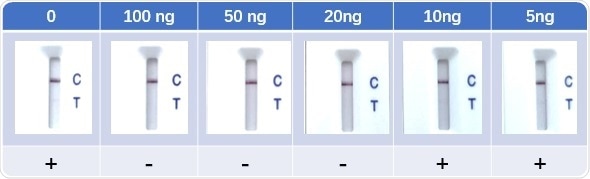
Figure 8. Lateral Flow Assay with spike-in neutralizing antibody serum samples (Cat No. SPD-M128). The anti-mouse IgG and RBD-his protein were coated to the control line and test line respectively. Colloidal gold-labeled ACE2 and mouse IgG was immobilized in a conjugate release pad. Image Credit: ACROBiosystems
References and Further Reading
- https://www.cdc.gov
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.