Over 40 years ago, there was an official declaration by the World Health Organization (WHO) that smallpox had been eradicated, thanks to the spread of the vaccine. With widespread vaccination programs held worldwide, today, several other diseases are on the precipice of being eradicated. The question on many minds is: could cervical cancer be next in line?
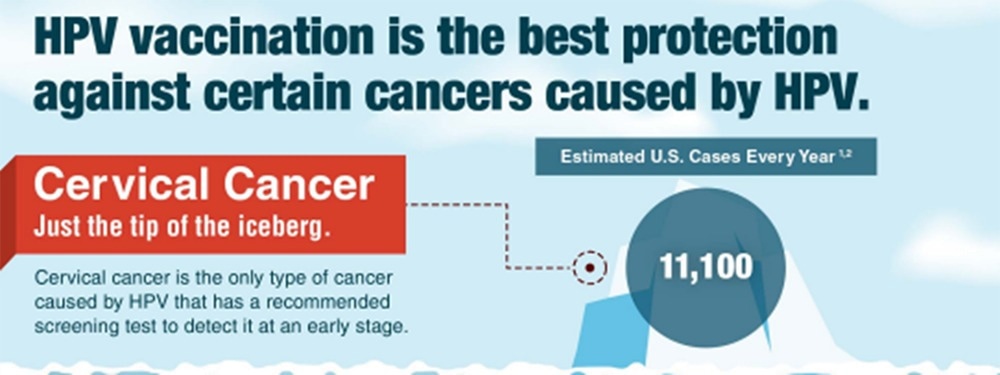
Image Credit: ACROBiosystems
Human papillomavirus (HPV) is a unique case for vaccinations: the virus itself accounts for over 95% of cervical cancer in women, which is itself the fourth most common type of cancer. The HPV vaccine was released in 2006, and, as the first to receive the vaccine are beginning to reach the average age at which cervical cancer is commonly onset, the success of HPV vaccines is becoming ever-clearer.
Vaccines are bringing the dream of eliminating cervical cancer within reach.”
General Tedros Adhanom Ghebreyesus, Director, WHO
Along with regular screening, the introduction of vaccine recommendations by the CDC within the US has led to a significant decline in cervical cancer cases. Among women between the ages of 20 to 24, cervical cancer incidence rates declined by over 65%, according to the American Cancer Society.
Across the globe, doctors have been advised to change their advice regarding HPV vaccines by the WHO Strategic Advisory Group of Experts (SAGE), with the emphasis being on paving the way for the prevention of cancer through vaccinations. Nonetheless, it must be acknowledged that HPV vaccines have been publicly available for some time. The question must be asked: what is the delay in eliminating cervical cancer?
The difficulty in this case is inequality of access.
In recent years, the integration of the HPV vaccine into immunization programs has slowed down as a result of various factors. These factors include limitations in resources, the growing anti-vaccination movement, and narrow indications of use in existing HPV vaccines. These factors are exemplified by a low global uptake of 13% - a significant drop from the 90% target set by WHO.
However, the recommended three-dose schedule has led to a significant issue. Countries that have the highest incidence rates of HPV infections (and of cervical cancer) have more limited resources and more regions with limited medical access. Vaccines requiring more than one dose are generally not as well-adopted and almost always end in a degree of non-compliance.

Image Credit: World Health Organization
HPV development should be focused on making the vaccine cheaper, less resource-intensive, and easier to administer, particularly if the world is to achieve the ambitious goals of the WHO. This scope includes expanding the indications of use into different age groups to cover women who fall outside the current age group receiving the vaccine and who lack access to HPV vaccines.
Similarly, vaccination rates would be significantly improved by a one-dose schedule by eliminating the financial or logistical challenges in implementing HPV vaccines into vaccination programs. Further cohort studies are required to ensure that the change in dosing recommendations would maintain its current efficacy.
What do HPV vaccine manufacturers have left to do?
The remarkable success of the HPV vaccine unfortunately does not mean that the current vaccines are perfect. The feasibility of implanting a one-dose schedule is different for every country, as each offers different vaccination options. Though developing a sustainable, one-dose HPV vaccine that has been comprehensively evaluated is a significant challenge that could take years, it may also be the right path toward eliminating the global risk of cervical cancer.
A major bottleneck, as with most virus research, is the challenges posed by cultivating viruses in vitro. The virus is a critical component in neutralizing antibody titer studies, despite the substantial safety concerns for the scientists and laboratory involved. The number of antibodies produced by the immune system is counted by the key evaluation study, offering a direct indicator as to the efficacy of new and potential vaccines.
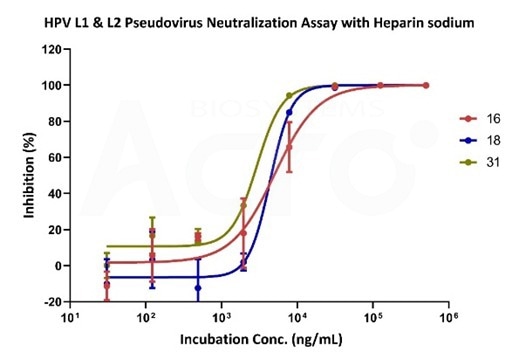
Neutralizing data of sodium heparin using various HPV-subtype pseudoviruses. Image Credit: ACROBiosystems
However, it must be noted that the majority of laboratories are not equipped with suitable equipment to store and manipulate viruses. This limitation in resources ultimately means that using the actual virus is not always possible. On the other hand, pseudoviruses are virus particles that express the same proteins on their membrane surfaces as actual viruses do.
Pseudoviruses are designed to mimic viral entry into the host cell without the pathogenic genetic sequences or autonomous replication ability.
Evaluation of HPV vaccines can be performed more frequently with the availability of HPV pseudoviruses, helping boost the possibility of developing a sustainable, one-dose HPV vaccine and eradicating cervical cancer.
About ACROBiosystems’ ViruStop & Pseudovirus services
ACROBiosystems is continuously observing and following the development of various viruses and infectious diseases worldwide to rapidly offer a wide range of products designed to accelerate vaccine development and other anti-viral therapeutics under the ViruStop brand. This array includes a wide-ranging virus antigen bank such as influenza, SARS-COV-2, Ebola, Nipah, and many others.

Image Credit: ACROBiosystems
Under ViruStop, ACROBiosystems offers a wide array of HPV pseudoviruses, including subtypes 6, 11, 16, 18, 31, 33, 45, 52, and 58. Pseudovirus services are also available, including custom reporter pseudovirus development (both enveloped and non-enveloped viruses) in addition to other technical evaluation services such as evaluation of anti-HPV drugs, screening, neutralizing antibody assays, and other vaccine efficacy assessments.
Explore the ViruStop platform and see how ACROBiosystems can help to accelerate your infectious disease research or vaccine development process.

Image Credit: ACROBiosystems
About ACROBiosystems
ACROBiosystemsis a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.