The SARS-CoV-2 virus contains four structural proteins: spike protein (S), nucleocapsid protein (N), envelop protein (E), and membrane protein (M). Among them, S protein is considered to be crucial to the infection process as it recognizes the target receptor, which mediates the fusion between the virus and the target cell membrane.
After infection, S protein is also the main target for neutralizing antibodies. Therefore, in comparison to the other three structural proteins, S protein has garnered much more attention in the development of COVID-19 vaccines and therapeutic antibodies.
S protein consists of two subunits, S1 and S2. Throughout the process of infection, the S1 subunit connects to the ACE2 receptor while the S2 subunit anchors the S protein to the host cell membrane controls the fusion of the virus envelope, and the host cell membrane.
There are two essential domains in S1 subunits, known as Receptor Binding Domain (RBD) and N-Terminal Domain (NTD). It is known that RBD is responsible for the process of binding to ACE2. S2 has three domains called Heptad Repeat (HR), Central Helix (CH), and Connector Domain (CD) respectively. Furthermore, there is a furin cleavage site at S1/S2 (Fig. 1).1
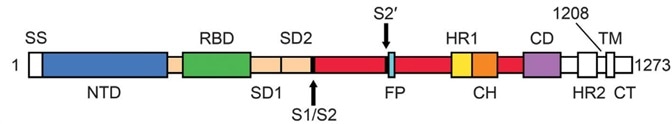
Figure 1. The sequence of Spike protein.
The S protein protrudes from the viral surface as a homotrimer with two different conformations, prefusion, and postfusion (Fig. 2).2 The binding of RBD and ACE2 initiates the structural change, which dissociates the S1 and S2 subunits and converts the S2 subunit into a highly stable postfusion conformation: scientists discovered that the S protein is structurally unstable. Moreover, it will change from prefusion to postfusion conformation spontaneously even without binding, which poses a massive challenge to the advancement and progress of vaccines and therapeutic antibodies.
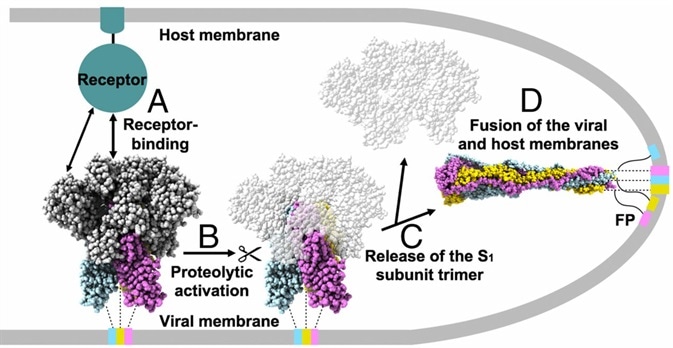
Figure 2. Proposed model of coronavirus entry.
Considering how important the S protein is, it will be crucial to the stable and consistent trimeric S protein reagent for the progress of vaccines, therapeutic antibodies, or serological test kits. After completing copious amounts of work, scientists discovered that 2 proline mutations (S-2P) of the S2 subunit stabilize the S protein conformation, which for the most part will remain at the prefusion state. This article will guide you through various scenarios that illustrate how the recombinant spike trimer protein could help in the subsequent part.
Scenario 1: Structural biology research
It is key to study the three-dimensional structure of the S protein, particularly for an understanding of receptor recognition and membrane fusion process. The instability of the conformation of natural S protein creates a lot of trouble. S-2P mutations stabilize the protein. Scientists can utilize recombinant S-2P protein samples to examine structural biology (Fig. 3).1
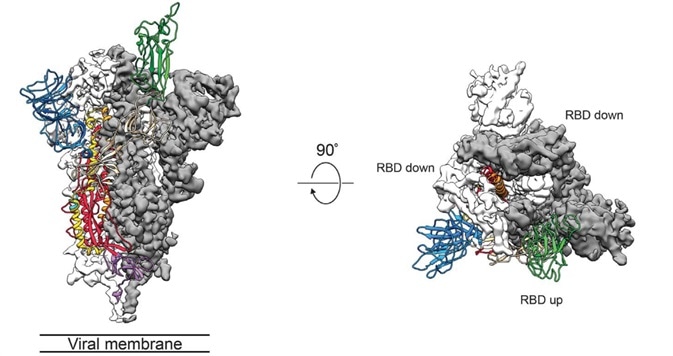
Figure 3. Cryo-EM of Spike protein.
Scenario 2: Vaccine development
Typically, a vaccine consists of an agent that is similar to a disease-causing microorganism. The agent triggers the body's immune system to generate neutralizing antibodies to protect from the agent that it may interact with in the future. Presently, there are over 300 COVID-19 vaccines in various stages of development, globally. Except for the inactivated vaccines, most vaccines utilize S protein as the immunogen.
The vaccine established using S trimer protein in the prefusion state could help protect humans from SARS-CoV-2 attack since the virus is in the prefusion state when they bind to the target cell. It was discovered that a majority of companies use S-2P in their vaccine design including Moderna, Johnson&Johnson, Pfizer, and Novavax. This discovery demonstrates the importance of the S protein conformation in the development of a vaccine (Fig. 4).
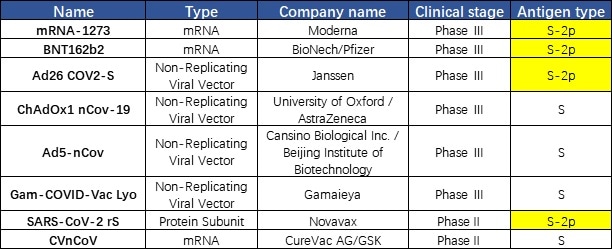
Figure 4. Vaccines under development targeting S protein.
Scenario 3: Serological diagnostic kit development
NIH utilized the S-2P protein for the development of their diagnostic kit. They used a various methods to characterize their recombinant S trimer protein to ensure the appropriate protein function including SEC-MALS, SDS-PAGE, and negative stain for electron microscopy (Fig. 5).
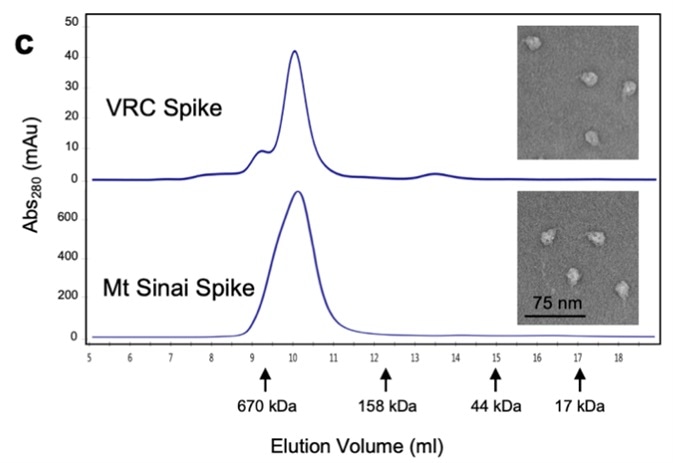
Figure 5. Characterization of trimeric S protein.
When evaluating the immunogenicity of the COVID-19 vaccine, ELISA is typically used to measure the titers of particular antibodies against the S protein. S-2P protein is commonly used in these ELISA assays for antibody detection.4
Scenario 4: Neutralizing antibody screening
At present, RBD protein is used to screen out a majority of the therapeutic antibodies in the clinical stage. However, it really can provide additional benefits to using S-2P protein for screening. In addition to the antibodies attaching themselves to RBD, you can obtain antibodies that become affixed to NTD and S2 at the same time. These antibodies could be combined and potentially offer a better therapeutic outcome (Fig. 6).5
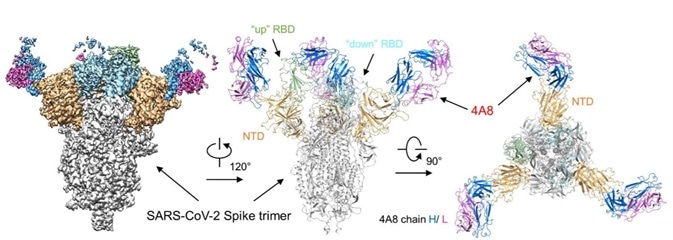
Figure 6. Cryo-EM of S protein and NTD domain.
Recently, scientists discovered 4 extra proline mutations of the S2 subunit (S-6P) that can stabilize the prefusion state of the S trimer protein further. In comparison to S-2P, S-6P should have improved performance in all of the scenarios above (Fig. 7).6
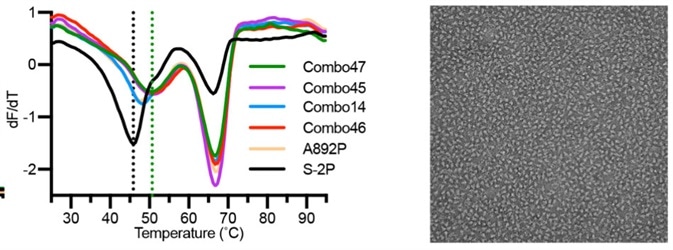
Figure 7. The stability and negative staining results of S-6P protein.
ACROBiosystems has specifically designed a highly active S trimer protein. This is the only trimeric S protein verified on the market. The SEC-MALS and negative-stain EM data show that ACRO's S protein is in the proper trimer form under physiological conditions, and purity exceeds 90%. The ELISA data illustrates that the S trimer protein attaches to human ACE-2 protein and anti-SARS-CoV-2 neutralizing antibody well.
Assay data
Cat. No. SPN-C52H9 SARS-CoV-2 S protein, his tag, super stable trimer (MALS & NS-EM verified)

Figure 8. The purity of SARS-CoV-2 S protein, His Tag, Super stable trimer (Cat. No. SPN-C52H9) was more than 90% verified by SDS-PAGE under reducing (R) condition. The molecular weight was around 550-660 kDa confirmed by SEC-MALS. The particles are similar in size and appearance to SARS-CoV-2 trimers reported in published literature verified by negative stain electron micrography.
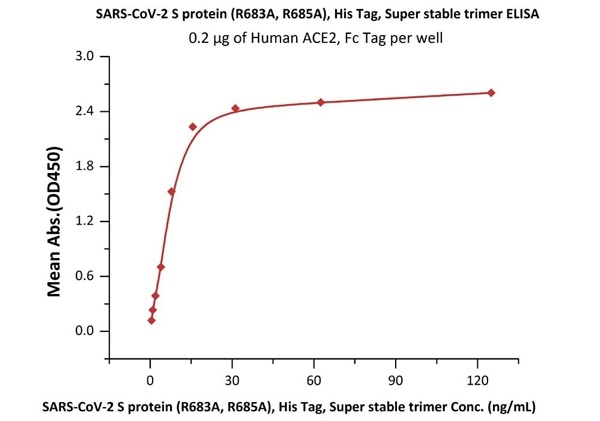
Figure 9. Immobilized Human ACE2, Fc Tag (Cat. No. AC2-H5257) at 2 μg/mL (100 μL/well) can bind SARS-CoV-2 S protein (R683A, R685A), His Tag, Super stable trimer (Cat. No. SPN-C52H9) with a linear range of 0.5-16 ng/mL.
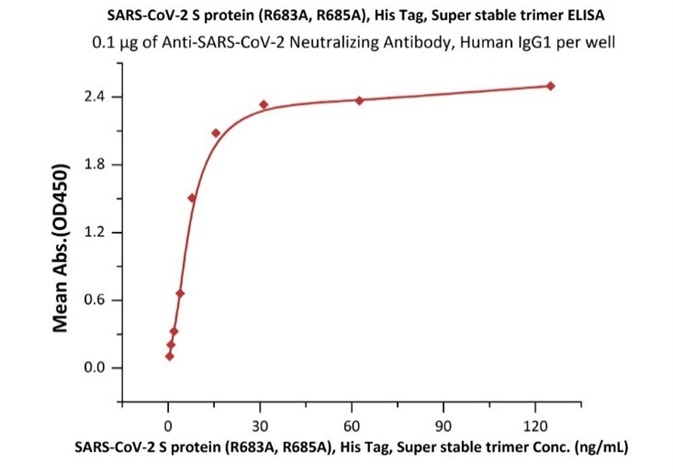
Figure 10. Immobilized Anti-SARS-CoV-2 Neutralizing Antibody, Human IgG1 (Cat. No. SAD-S35) at 1 μg/mL (100 μL/well) can bind SARS-CoV-2 S protein, His Tag, Super stable trimer (Cat. No. SPN-C52H9) with a linear range of 0.3-10 ng/mL.
Image References
- Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation
- Tectonic conformational changes of a coronavirus spike glycoprotein promote membrane fusion
- Standardization of enzyme-linked immunosorbent assays for serosurveys of the SARS-CoV-2 pandemic using clinical and at-home blood sampling
- An mRNA Vaccine against SARS-CoV-2 — Preliminary Report
- A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2
- Structure-based design of prefusion-stabilized SARS-CoV-2 spikes
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.