The antibody test - also referred to as the serology test – can provide a crucial supplementary diagnosis of COVID-19. As well as the nucleic acid test, the antibody test can assist with precision diagnoses as well as characterization of the spread and prevalence of the disease.
An antibody test can identify whether or not a person has been exposed to SARS-CoV-2 and whether or not they have developed immunity against SARS-CoV-2 infection. This test is generally is better suited for vaccine development and public health surveillance.
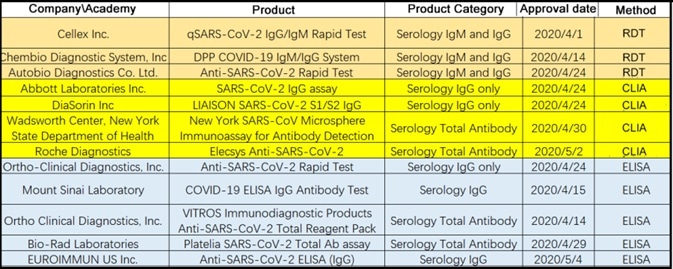
The FDA has, so far, issued Emergency Authorization Use to twelve antibody tests, including four chemiluminescent assays, three colloidal gold assays, and five ELISA assays (see Table 1).
Table 1. Emergency Use Authorizations for COVID-19 by FDA. Source: ACROBiosystems
Due to demand and urgency, many tests are quickly developed before being made available on the market with only limited validation. To address this issue, scientists are assessing these assays by utilizing clinical samples. This article looks at the performance of the ELISA assays, specifically.
Beijing Wantai Biological Pharmacy Enterprise Co. Ltd. has created three enzyme-linked immunosorbent assay (ELISA) kits, designed to work with the total antibodies (Ab), IgM, and IgG of SARS-CoV-2 respectively.
The ELISA for Ab has been developed based on the double-antigens sandwich ELISA, employing the mammalian cell-expressed recombinant receptor binding domain (RBD) of SARS-CoV-2’s spike protein and the HRP-conjugated antigen.
The IgM μ-chain capture ELISA was utilized to identify the IgM antibodies via the same HRP-conjugated antigen. The IgG antibodies were quantified with an indirect ELISA kit based on a recombinant nucleocapsid protein.
This study involved 173 patients. The seroconversion rate for Ab, IgM, and IgG was found to be 93.1% (161/173), 82.7% (143/173), and 64.7% (112/173), respectively.
Twelve patients remained seronegative for Ab testing, though this was possibly due their samples all being collected during the early stages of illness. The specificity of assays for Ab, IgM, and IgG was established as 99.1% (211/213), 98.6% (210/213), and 99.0% (195/197) using samples obtained from healthy people.
The different ELISA tests’ performances were also analyzed, alongside the nucleic acid test in samples with varying durations of illness. As the length of illness increased, the sensitivity of ELISA tests’ continued to increase as well, surpassing the nucleic acid test’s sensitivity after day eight.
Overall, use of both the nucleic acid test and ELISA test in conjunction with one another is believed to markedly enhance the sensitivities of COVID-19 diagnosis in various stages.
A group from Denmark has also examined the performance of three commercially available ELISA kits, including the Wantai Ab ELISA kit mentioned earlier, and two ELISA kits from Euroimmun (IgG and IgA respectively). The latter two tests received EUA from the FDA in May (Table 1).
The IgG or IgA antibodies against SARS-CoV-2 spike protein subunit 1 (S1) can be detected via an indirect ELISA format.
As can be seen in the ELISA data distribution figure (Figure 1), both the positive and negative data points were different for the Wantai total Ab assay, which possessed a cut-off value above every control serum sample, thus allowing unequivocal interpretation. On the contrary, the Euroimmun IgA and IgG assay data exhibited a less distinct separation.
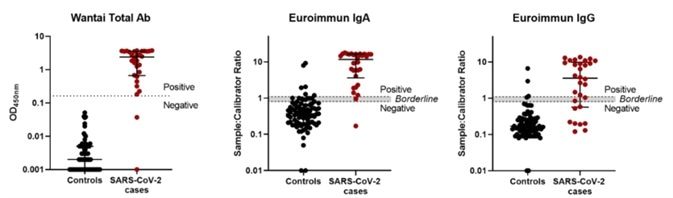
Figure 1. SARS-CoV-2 antibody ELISA assay performance. Image Credit: ACROBiosystems
The Wantai total Ab ELISA displayed improved sensitivity and specificity when compared to both Euroimmun IgA and IgG ELISAs (see Table 2). This markedly lower sensitivity for SARS-CoV-2-specific IgG detection can be seen to be in agreement with the observations outlined in the previous study which discussed the Wantai IgG ELISA kit.
Table 2. Analytical sensitivities and specificities for ELISA kits. Source: ACROBiosystems
There is a possibility that generally reduced sensitivity of SARS-CoV-2 IgG ELISAs could be a more widespread occurrence rather than being manufacturer dependent, and this warrants further exploration.
As well as offering lower sensitivities, the Euroimmun IgA and IgG ELISAs are also more likely to cross-react with negative serum samples. Differing performances between these assays could be somewhat clarified by the ELISA format and the antigens used within certain instances.
Nucleocapsid and spike proteins are important reagents for antibody detection kits, which has the potential to considerably affect assay performance.
ACROBiosystems has successfully developed S1, N, and wild type RBD, alongside mutant RBD proteins which possess various tags including His, Fc, mFc, His and Avi, all of which are well suited for COVID-19 serological test development.
These proteins are suitable for application as part of a chemiluminescence method, colloidal gold method, and ELISA method. ACRO has received a great deal of positive feedback for its range of high quality SARS-CoV-2 antigen proteins.
Cat. No.: NUN-C51H9
Product: SARS-CoV-2 (COVID-19) Nucleocapsid protein, his tag
The sensitivity of N protein binding to Anti-N mAb is 0.02 ng/mL as verified by ELISA.
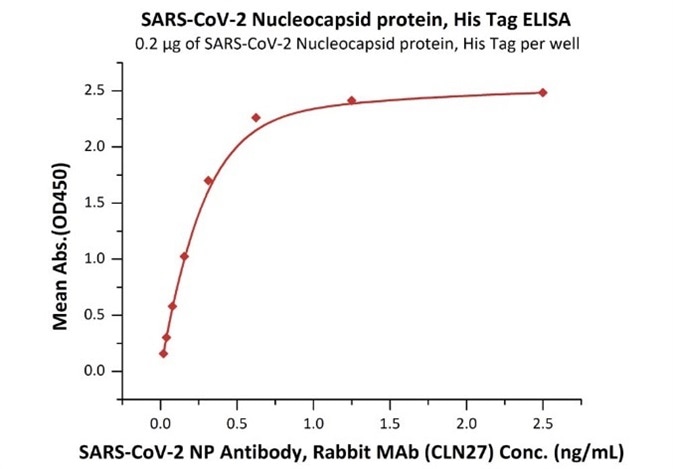
Immobilized SARS-CoV-2 Nucleocapsid protein, His Tag (Cat. No. NUN-C51H9) at 2 μg/mL (100 μL/well) can bind SARS-CoV-2 NP Antibody, Rabbit MAb (CLN27) with a linear range of 0.02-0.3 ng/mL. Image Credit: ACROBiosystems
Cat. No.: S1N-C52H4
Product: SARS-CoV-2 (COVID-19) S1 protein, his tag (MALS verified)
The sensitivity of S1 protein binding to ACE2 protein is 0.2 ng/mL as verified by ELISA.
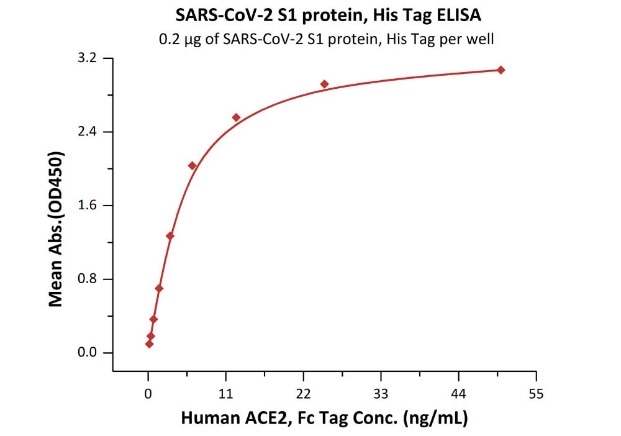
Immobilized SARS-CoV-2 S1 protein, His Tag (Cat. No. S1N-C52H4) at 2 μg/mL (100 μL/well) can bind Human ACE2, Fc Tag (Cat. No. AC2-H5257) with a linear range of 0.2-6 ng/mL. Image Credit: ACROBiosystems
Cat. No.: SPD-C52H3
Product: SARS-CoV-2 (COVID-19) S protein RBD, his tag (MALS verified)
The sensitivity of RBD protein binding to ACE2 protein is 0.008 ng/mL as verified by ELISA.
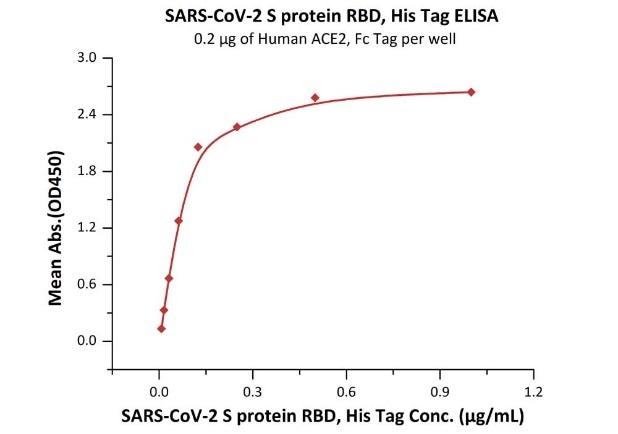
Immobilized Human ACE2, Fc Tag (Cat. No. AC2-H5257) at 2 μg/mL (100 μL/well) can bind SARS-CoV-2 S protein RBD, His Tag (Cat. No. SPD-C52H3) with a linear range of 0.008-0.125 μg/mL. Image Credit: ACROBiosystems
References
- Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019
- Evaluation of nine commercial SARS-CoV-2 immunoassays
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.