Confirmed COVID-19 cases worldwide have risen to over 6 million, and scientists are continually moving forward in their efforts to develop treatments and vaccines that hinder the spread of the pandemic and reduce the damage caused by the disease.
Hundreds of projects are currently taking place globally that are focused on the development of a vaccine for the COVID-19 virus. Eight candidate vaccines have been tested in clinical trials, as of May 31,1 but it is likely that the vaccine will not be available until 2021.
However, the development cycle of neutralizing antibodies is fairly short, leading many experts to believe that the efforts of companies developing antibody drugs are equally essential.
Antibodies differ from vaccines or antiviral drugs in their potential to both protect against and treat viral infections. With high specificity and ease of upscaling being clear advantages, the development of neutralizing antibodies is drawing a great deal of attention around the world.
Sunney Xie’s group recently showcased their work around the screening of neutralizing antibodies against SARS-CoV-2. This was published in leading journal ‘Cell’.
The group hypothesized that enriched B cell clonotypes were more likely to yield high-affinity SARS-CoV-2 binding alongside neutralizing antibodies. They began by conducting a high-throughput sequencing of single B cells collected from convalescent patients.
Initially, the group only found a single RBD-binding mAb that possessed a weak virus neutralization ability. In order to optimize the method, the group utilized RBD and S protein pre-coupled magnetic beads to enrich the RBD binding B cells (Figure 1).
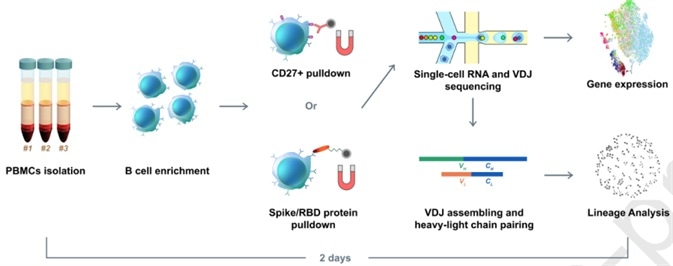
Figure 1. Illustration of RBD binding B cells enrichment using RBD and S protein pre-coupled magnetic beads. Image Credit: ACROBiosystems
A total of 169 ideal candidates were chosen based on a predefined set of criteria. Following additional screening with ELISA and SPR, 149 S-binding mAbs were identified, among which 70 mAbs were found to bind to the RBD.
All ELISA-positive mAbs were further screened for neutralizing ability via a SARS-CoV-2 pseudovirus system, and it was found that amongst the neutralizing mAbs, seven of them displayed effective neutralization ability with an IC50 of less than 0.05 μg/mL (Figure 2A).
To properly validate their neutralization potential versus the authentic virus, a plaque reduction neutralization test was performed which found that BD-368-2 exhibited the highest potency against the authentic virus, possessing an IC50 of 15 ng/mL (Figure 2B).
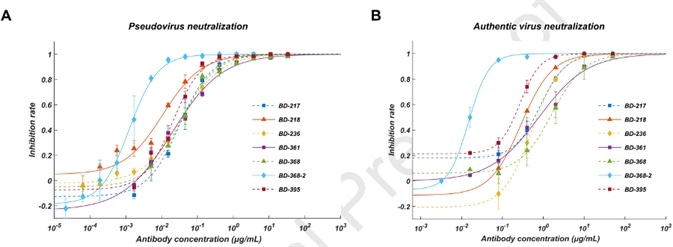
Figure 2A. Pseudovirus neutralization result; B. Authentic virus neutralization result. Image Credit: ACROBiosystems
To further evaluate whether or not these neutralizing mAbs could effectively serve as therapeutic interventions and offer prophylactic protection against SARS-COV-2 in vivo, the group tested the neutralization efficacy of BD-368-2 on hACE2 transgenic mice that had been infected with SARS-CoV-2.
This process confirmed that the BD-368-2 demonstrated high therapeutic and prophylactic efficacy in vivo (Figure 3).
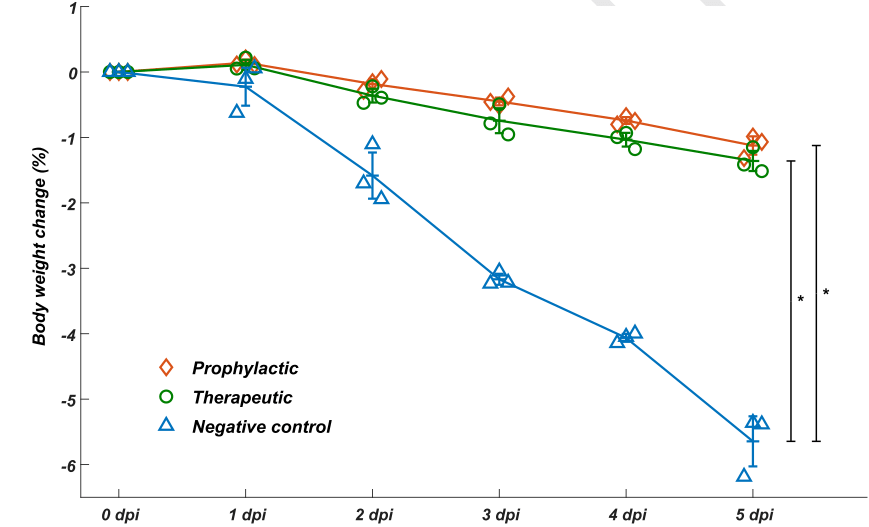
Figure 3. BD-368-2 exhibits high therapeutic and prophylactic efficacy in vivo. Image Credit: ACROBiosystems
By examining the Cry-EM results (see Figure 4 below), it can be seen that the S ectodomain adopts an asymmetric conformation with the RBD in one protomer (mol A) adopting an ‘up’ position. In contrast, the other two RBDs (mol B and C) adopt ‘down’ positions.
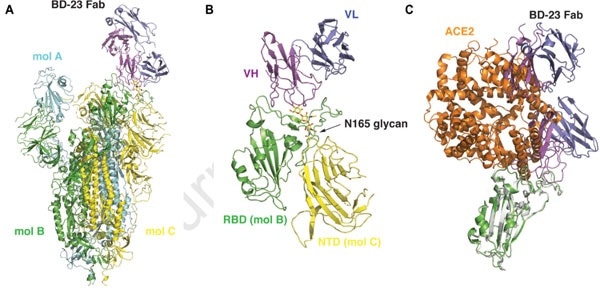
Figure 4. 3D reconstruction results of Cryo-EM. Image Credit: ACROBiosystems
This 3D reconstruction displays a single BD23-Fab which has been observed per S trimer. This binds the ‘down’ RBD in protomer B, while only BD-23’s heavy chain variable domain is involved in binding to the RBD.
Bioinformatic-based selection methods were further employed in the study, finding that mAbs capable of neutralizing antibodies which shared a highly similar CDR3H structure with SARS-CoV, also displayed a high percentage of neutralization potency for SARS-CoV-2. Therefore, structure analysis is able to assist in increasing the efficiency of neutralizing antibody screening.
A key feature of this study is the use of RBD and S protein pre-coupled magnetic beads to enrich B cell clonotypes. According to an interview with Sunney Xie - the author of the paper - the pre-coupled magnetic beads have the potential to help increase the efficiency of B cell enrichment twenty fold.3
ACROBiosystems has developed high-quality S1 protein and RBD protein pre-coupled magnetic beads, in order to fill this gap in the market. These pre-coupled magnetic beads offer convenience, minimum non-specific binding alongside validated protocols.
This new, ready-to-use product offers time savings which will eventually lead to acceleration in therapeutic antibody development.
Quantification of the pre-coupled RBD protein
Static adsorption experiments have shown that RBD protein-coupled magnetic more are able to capture the anti-SARS-CoV-2 S1 antibody, while the RBD load is greater than 40 μg/mg magnetic beads.
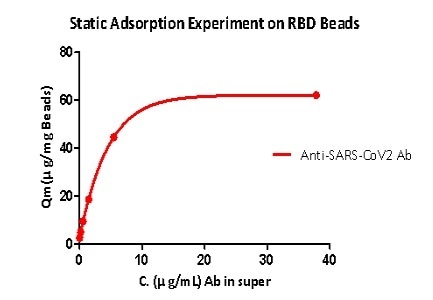
Figure 5. Static adsorption experiment on RBD beads. Image Credit: ACROBiosystems
Binding test of RBD pre-couple magnetic beads
Strict QC tests were undertaken with these pre-coupled beads. This process begins with 0.1 mg beads being added to a tube. The supernatant is then removed after washing, before 100 μL of the ACE2 protein or antibody (10 μg/mL ~0.039 μg/mL) was added to the beads for a 1 hour incubation period. Next, a fluorescent-labeled secondary antibody was utilized for detection.
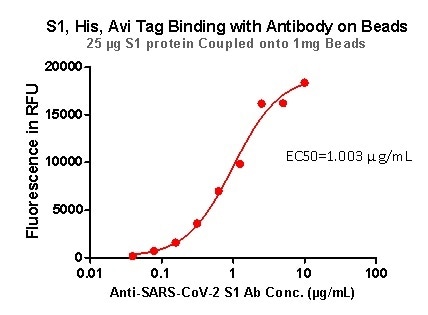
Figure 6. S1,His,Avi Tag Binding with Antibody on beads. Image Credit: ACROBiosystems
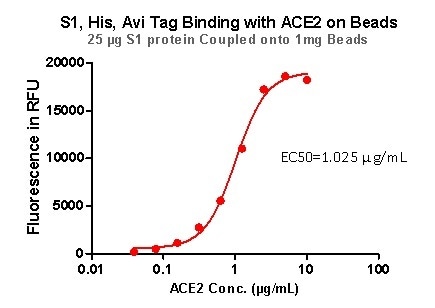
Figure 7. S1,His,Avi Tag Binding with ACE2 on Beads. Image Credit: ACROBiosystems
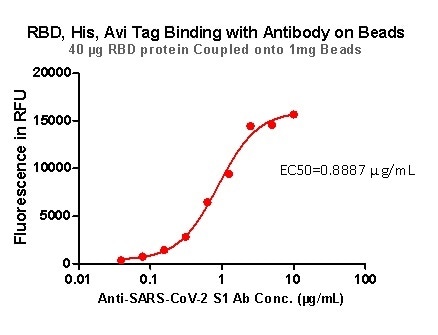
Figure 8. RBD,His,Avi Tag Binding with Antibody on beads. Image Credit: ACROBiosystems
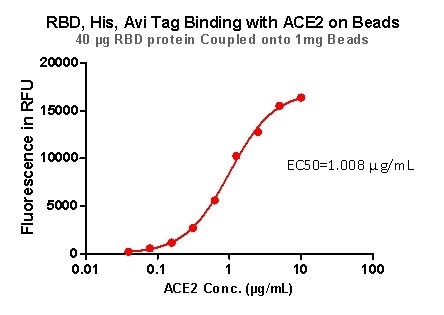
Figure 9. RBD,His,Avi Tag Binding with ACE2 on beads. Image Credit: ACROBiosystems
It is possible to use pre-coupled magnetic beads for sample enrichment, immunocapture, screening and biopanning. The quality of both the microsphere and the coupled protein have the biggest impact on the performance of these pre-coupled beads.
References
- https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker
- Cao, Y., et al., Potent neutralizing antibodies against SARS-CoV-2 identified by high-through put single-cell sequencing of convalescent patients’ B cells, Cell (2020), doi: https://doi.org/10.1016/j.cell.2020.05.025.
- https://finance.sina.com.cn/wm/2020-05-21/doc-iirczymk2863827.shtml?cre=tianyi&mod=pcpager_focus&loc=1&r=9&rfunc=61&tj=none&tr=9
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.