The pandemic which is due to the emerging coronavirus SARS-CoV-2 brings about a serious global public health emergency in urgent need of therapeutic and prophylactic interventions.
Scientists working to stop the COVID-19 pandemic think it will be viable to isolate monoclonal antibodies (mAbs) in SARS-CoV-2 infected patients and discover which antibodies are the most potent in stopping a coronavirus infection, before synthetically making large volumes of identical copies of these proteins in order to treat COVID-19.
A team of Chinese scientists recently published a research paper1 on the preprint server for Biology, bioRxiv, demonstrating the isolation and characterization of mAbs derived from single B cells of eight SARS-CoV-2 infected individuals. The key points of this study are summarized here:
1. Serial dilutions of plasma samples taken from eight SARS-CoV-2 infected subjects were examined for binding to RBDs from SARS-CoV, SARS-CoV-2, and MERS-CoV. The plasma samples showed no cross-reactivity was detected against SARS-CoV RBD and MERS-CoV RBD, but binding activity was seen to SARS-CoV-2 RBD. The results indicate that the RBD-based antibody response is viral species-specific, as seen in Fig 1.
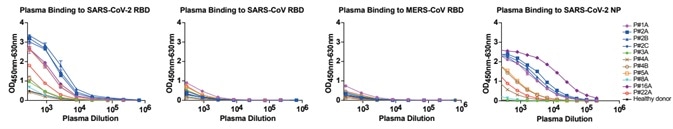
Figure 1. Analyses of plasma responses specific to SARS-CoV-2. Image Credit: ACROBiosystems
2. As seen in Fig 2, Flow cytometry was employed to analyze SARS-CoV-2-specific B cell responses and identify B cells recognizing fluorescent-labeled RBD probes. RBD-binding B cells were isolated further into single cell suspension for cloning and then the 206 mAbs that bound to SARS-CoV-2 RBD were obtained.
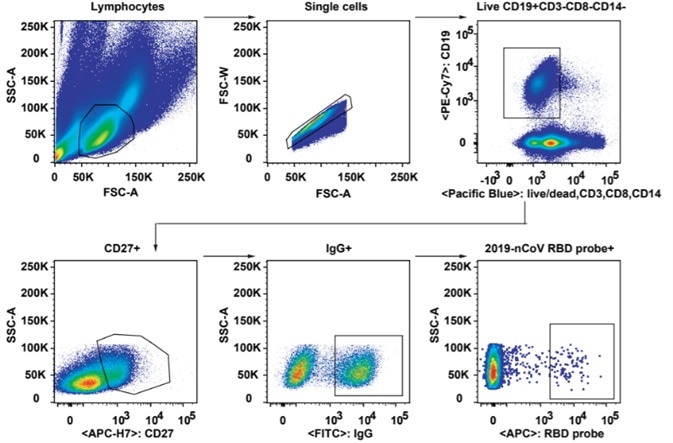
Figure 2. Gating strategy for analysis and isolation of SARS-CoV-2 RBD-specific memory B cells. Image Credit: ACROBiosystems
3. Based on their distribution on the phylogenetic tree, 18 antibody clones were chosen and their binding to SARS-CoV-2 RBD was detected by utilizing surface plasmon resonance (SPR). While none of the antibodies showed cross-binding with SARS-CoV RBD and MERS-CoV RBD, the dissociation constants of the antibodies ranged from 10-8 to 10-9 M, as seen in Fig 3.
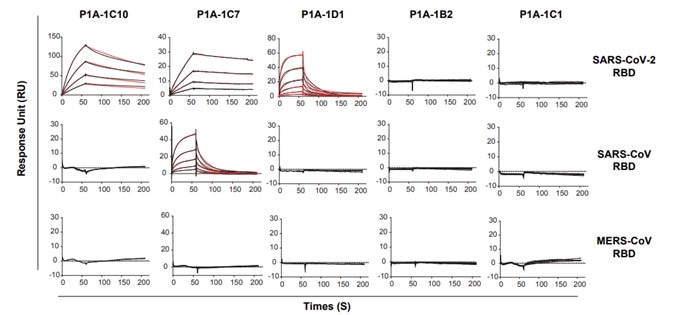
Figure 3. Binding kinetics of selected mAbs with SARS-CoV-2 RBD, SARS-CoV RBD and MERS-CoV RBD measured by SPR. Image Credit: ACROBiosystems
4. The 18 antibodies were measured for competition with ACE2 for binding to the SARS-CoV-2 RBD. While many antibodies exhibited strong binding to ACE2, they had only limited competing power with ACE2, indicating that binding affinity is not predictive of ACE2 competing capacity, as seen in Fig 4.
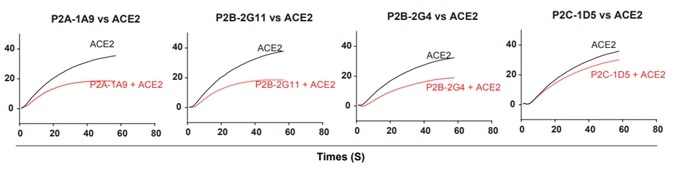
Figure 4. Antibody and ACE2 competition for binding to SARS-CoV-2 RBD measured by SPR. Image Credit: ACROBiosystems
5. Antibody neutralizing activities were examined against pseudoviruses bearing the Spike protein of SARS-CoV-2. The neutralizing activities correlated well with the ACE2 competing capacity, especially the most potent antibodies (299 P2C-1F11 and P2B-2F6) out-competed ACE2 with near to 100 % efficiency, suggesting that blocking the RBD and ACE2 interaction is a helpful surrogate for antibody neutralization, as seen in Fig 5.
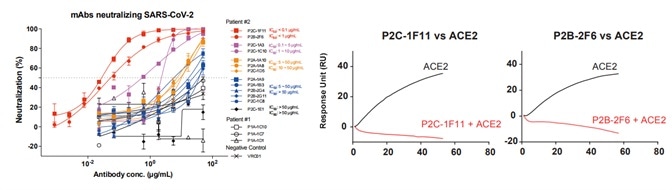
Figure 5. Antibody neutralization analyzed by pseudovirus. Image Credit: ACROBiosystems
The potent and diverse neutralizing antibodies identified in this study are promising candidates for therapeutic and prophylactic SARS-CoV-2 interventions. Presently, the scientists are performing preclinical studies in the hopes of translating the identified mAbs into anti-COVID-19 drugs.
As suggested by the research paper, blocking the ACE2 and RBD interaction is a helpful surrogate for antibody neutralization. Yet, the low-throughput of SPR instruments limits the screening of big volumes of candidate drugs including small molecules and neutralizing antibodies.
ACROBiosystems developed the ‘SARS-CoV-2 Inhibitor Screening Kit’ to solve this problem. This uses the colorimetric ELISA platform to measure the inhibitory effect of potential inhibitors on blocking the binding between immobilized SARS-CoV-2 S protein RBD and in-house developed biotinylated human ACE2 protein, as seen in Fig 6.
This product would allow researchers to perform high-throughput screening of SARS-CoV-2 inhibitors and to speed up the development of therapeutics against COVID-19.
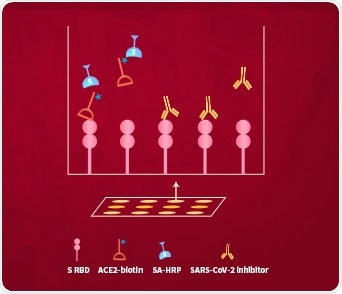
Figure 6. The schematic diagram of the ‘SARS-CoV-2 Inhibitor Screening Kit’. Image Credit: ACROBiosystems
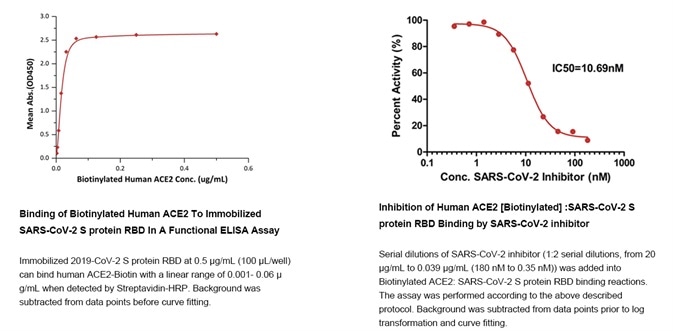
Figure 7. Data derived from using the ‘SARS-CoV-2 Inhibitor Screening Kit’. Image Credit: ACROBiosystems
Reference
1. Potent human neutralizing antibodies elicited by SARS-CoV-2 infection.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.