Over 10 billion doses of COVID-19 vaccines have already been administered globally, but in the months since the emergence of the Omicron variant (B.1.1.529), new challenges have arisen along with the rates of infection.
In the United States, the average new cases and daily fatalities linked to Omicron have already surpassed those during the Delta variant’s peak in September 2021.
These ongoing outbreaks of a highly infectious new variant have prompted significant research into improving existing vaccines and developing new vaccines.
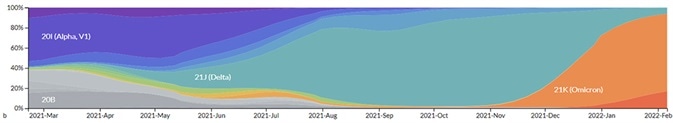
Genomic epidemiology of novel coronavirus - Global subsampling. Image Credit: ACROBiosystems
Increasing breakthrough infection cases
Spike protein has a central role in COVID-19’s infection pathway, binding to human cell surface receptors to prompt cell fusion and invasion.
Existing research has demonstrated that mutations in spike proteins can bolster viral replication and immune avoidance, essentially making the virus more resistant to vaccines.
The Omicron variant features more than 30 mutations in its spike protein alone, including important mutation sites present in all four current variants of concern. These mutations serve to greatly enhance its immune escape capabilities.
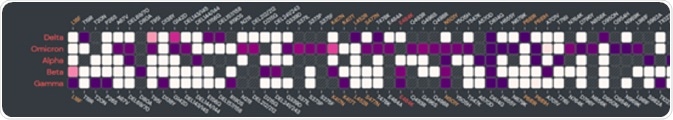
Image Credit: ACROBiosystems
Studies have shown that even in vaccinated people, the titer of neutralizing antibodies is significantly reduced against Omicron. This means that the breakthrough infection may occur even in cases where two doses of vaccine have been administered.
Roy Chemaly, an Infection and Disease Control Specialist from MD Anderson USA, pointed out that the number of breakthrough infection cases due to Omicron has considerably exceeded the breakthrough cases of Delta.
Developing new vaccines which offer sufficient protection against the Omicron variant has, therefore, become urgent.
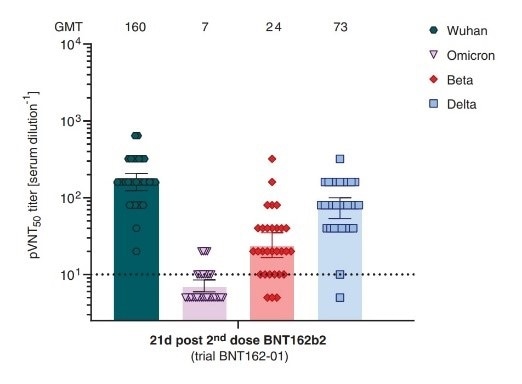
Neutralizing titers of sera from BNT162b2 vaccinated recipients. Image Credit: ACROBiosystems
Omicron vaccines progress
Vaccine manufacturers recognize this need and are investing substantial amounts of time and resources into vaccines that are able to protect against this highly transmissible variant.
For example, Pfizer Inc. and BioNTech are currently developing Omicron-based vaccines due to launch soon, while the U.S. vaccine company Moderna is developing a new generation of COVID-19 vaccine - mrna-1273.529 (also referred to as mRNA-Omicron).
This vaccine is designed to be administered as a booster shot and is now in clinical trials.
A team from the Department of Microbiology of the University of Hong Kong was the first research team in Asia to successfully isolate and cultivate the Omicron variant strain.
The team had announced that this isolated strain would be made available to the Chinese Center for Disease Control and Prevention, Sinovac biology and Sinophem for research and vaccine development on December 9th and December 10th, respectively.
Consino Bio had also announced its plan to develop a COVID-19 vaccine against the Omicron variant.
A range of early animal studies have indicated that Moderna’s Omicron-specific boosters offer no distinct advantage versus a third dose of current vaccines. The development and improvement of vaccines must, therefore, continue.
Polyvalent vaccines
The combination of COVID-19’s tendency for rapid transmission and its ability to continually mutate had led to the development of polyvalent vaccines - potentially the most effective defense in the struggle against COVID-19.
Vaccine development is subject to strict requirements around immunogenicity and effectiveness.
The detection of antigen content is, therefore, a vital step in this process, with the development of polyvalent vaccines necessitating the establishment of a method for the quantitative detection of mutated antigen.
To this end, ACROBiosystems has developed an Omicron-specific antibody (Cat.No. SPD-M305). This antibody will only bind to Omicron Spike RBD and does not bind to other COVID-19 strains.
The antibody’s primary features include high bioactivity, with its affinity between the Omicron-specific antibody and Omicron Spike RBD found to be 9.07 nM via a BLI assay. It is also highly pure, verified as more than 95% pure via SDS-PAGE.
The antibody is also ideal for detecting and quantifying mutant antigens as part of polyvalent and Omicron-based vaccine research and development.
Assay data
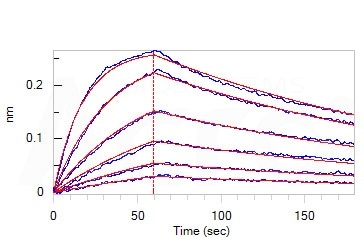
Loaded Monoclonal Anti-SARS-CoV-2 Spike RBD Antibody, Mouse IgG1 (Cat. No. SPD-M305) on AMC Biosensor, can bind SARS-CoV-2 Spike RBD, His Tag (B.1.1.529/Omicron) (Cat. No. SPD-C522e) with an affinity constant of 9.07 nM as determined in BLI assay (ForteBio Octet Red96e). Image Credit: ACROBiosystems
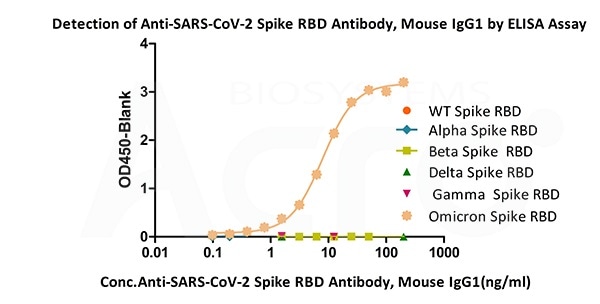
Immobilized SARS-CoV-2 Spike RBD (Omicron, Cat.No. SPD-C522e) can bind Anti-SARS-CoV-2 Spike RBD Antibody, Mouse IgG1(Cat.No.SPD-M305) with a linear range of 0.4-12.5 ng/mL. The antibody does not bind Spike RBD of WT (Cat.No. SPD-C52H1), Alpha (Cat.No. SPD-C52Hn), Beta (Cat.No. SPD-C52Hp), Delta(Cat.No. SPD-C52Hh) and Gamma (Cat.No. SPD-C52Hr). Image Credit: ACROBiosystems
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.