SARS-CoV-2 is mostly under control, thanks to vaccinations. However, cases and reinfections continue to rise globally despite the heavily immunized population. In particular, the emergence of the Omicron and its subvariants have triggered waves of the COVID pandemic.
Image Credit: ACROBiosystems
Populations immunized with the most recent mRNA vaccines are 20 to 130 times less vulnerable to Omicron mutations, according to a recent study published in medRxiv. Concerns regarding the effectiveness of current vaccines are growing as newer, more virulent strains start to test and evade the current immunity.
Numerous investigations have shown that the persistent emergence of novel, highly contagious variations significantly impairs resistance to infection over time. Booster doses are frequently advised to fight the declining vaccination efficacy. However, it is important to strike a balance between preventing serious illnesses and administering booster shots as often as is advised.
The VRBPAC’s (Vaccine and Related Biological Products Advisory Committee) main goal is to accomplish this. Similar to this, experts who supplied reports to the FDA (Food and Drug Administration) recommended that the new COVID-19 vaccine generation should focus on emerging strains and be administered annually.
This supports the idea that vaccine developers should keep working on next-generation COVID vaccines to protect against Omicron and any new strains.
Facing new variants and increases in reinfection, bivalent vaccines take the front seat
Booster shots based on BA.1 are effective against other strains, but the duration of their efficacy is becoming less definite. As BA.5, the virus strain with the most contagiousness to date, has taken over as the predominant strain, the FDA has advised that vaccine producers concentrate on creating a bivalent vaccine that includes BA.4/5 components.
Moderna released fresh clinical data on their Omicron-containing bivalent COVID booster candidate, mRNA-1273.214, in accordance with the FDA’s guidelines.
A booster dose of the new bivalent candidate produced 1.75 times more neutralizing antibodies against the Omicron variant than the original mRNA-1273 vaccination (Figure 1). They are also developing the bivalent COVID booster mRNA-1273.222, which should protect against the BA.4/5 variation.
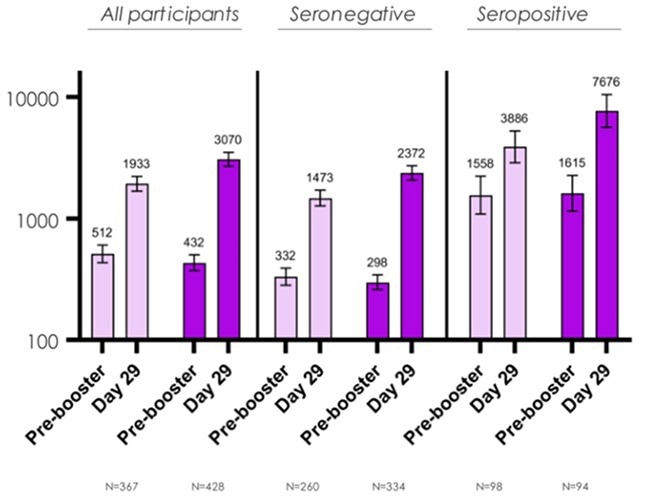
Figure 1. Comparison between mRNA-1273.214 and mRNA-1273. Image Credit: Moderna
Similar to this, Pfizer and BioNTech released encouraging information about the safety, tolerability, and immunogenicity of their bivalent vaccine. The bivalent vaccination combines the company's original COVID-19 vaccine with their candidate, which targets the spike protein of the Omicron BA.1 VOC.
Compared to their current COVID-19 vaccine, data from phase 2 and 3 clinical trials also showed a significantly stronger immune response against Omicron BA.1. In Figure 2, sera from participants aged 56 or older examined in the SARS-CoV-2 live virus neutralization experiment were able to effectively neutralize BA.4/5 despite having a 3-fold lower titer result than BA.1.
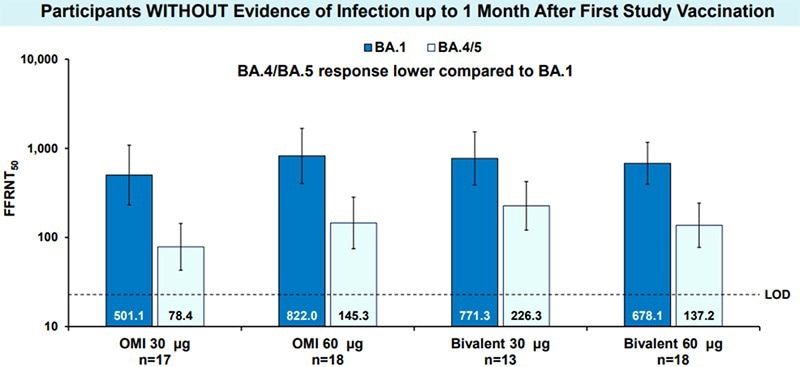
Omicron-containing Modified Variant Vaccines as 4th Dose Elicit Improved Omicron Neutralization Response. Image Credit: Pfizer and BioNTech
Future of COVID-19 vaccines
As new variants of SARS-CoV-2 continue to emerge, it is likely for the virus will coexist with humans for the time being. Many of the current next-generation mutant or bivalent vaccines are developed against the current dominant variant as booster shots. Although booster shots provide some level of immunity against infection, it is impractical to rely on them as a long-term fix.
Other new vaccine kinds, such as polyvalent, pan-coronavirus, or all-in-one vaccinations, are anticipated to be created in the near future. These will help to provide an all-incompassing defense against potential SARS-CoV-2 strains as well as other new coronaviruses.
It is essential for researchers and vaccine producers to keep up their efforts in the fight against COVID-19 and upcoming pandemics despite the formidable obstacles that are still present.
ACROBiosystems is dedicated to helping in the fight against COVID-19. To support the efforts of vaccine manufacturers, ACROBiosystems provides and continuously updates a series of core reagents as a comprehensive solution to develop mutant-based and bivalent vaccines.
Image Credit: ACROBiosystems
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.