The virus responsible for the current pandemic, SARS-CoV-2, is a positive-sense single-stranded RNA virus that is extremely contagious in humans. The viral envelope is comprised of a lipid bilayer, anchoring the membrane (M), envelope (E), and spike (S) structural proteins.
Consisting of an S1 and S2 subunit, the spike protein is a homotrimer. The globular S1 subunit facilitates receptor recognition, whereas the S2 subunit is involved in membrane fusion and anchors S into the viral membrane. Thus, the S protein is the protein vital to the coronavirus invasion of human host cells. S protein is also the primary antigen that leads to the host immune system producing neutralizing antibodies after infection.
Earlier this year, as claimed in an article published in Science, a team from UT Austin identified the cryo-electron microscopy structure of the 2019-nCoV S trimer in the prefusion conformation.1 As illustrated in Fig.1, prefusion S glycoproteins assume an equivalent mushroom-like homo-trimer architecture, of which the stem mainly consists of three S2 subunits, and the top cap is composed of three interwoven S1 subunits.3 Another team from Tsinghua University determined that the conformational switch of the receptor-binding domain(RBD) from the “down” to “up” position is essential for receptor binding (Fig. 2). Consequently, homo-trimer architecture is key to achieving the total functionality of the S protein.
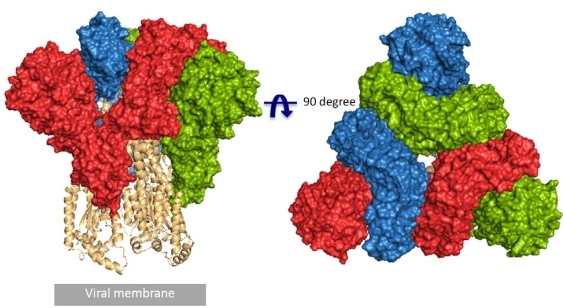
Figure 1. Front and top view of the trimeric coronavirus spike protein ectodomain obtained by cryo-electron microscopy analysis. Three S1 protomers (surface presentation) are colored in red, blue, and green. The S2 trimer (cartoon presentation) is colored in light orange. Image Credit: R.J.G. Hulswit, et al., Coronavirus Spike Protein and Tropism Changes. Adv Virus Res. 2016; 96: 29–57.
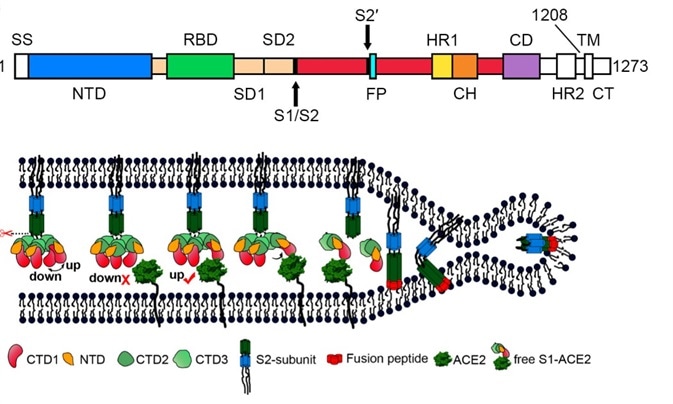
Figure 2. S protein mediates viral membrane and membrane fusion by binding to host cell receptor ACE2. Image Credit: Song W, Gui M, Wang X, Xiang Y (2018) Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog 14(8): e1007236.
The CoV spike (S) glycoprotein is the primary target for therapeutic antibodies, vaccines, and diagnostics. Therefore, identifying the proper trimeric structure and function of the recombinant S protein reagent is key. Strict quality control is necessary for terms of the molecular weight and aggregation state. Recently, a work emerging from NIH, a scientist utilized three methods including analytical size exclusion chromatography, SDS-PAGE, and transmission electron microscopy to determine that the recombinant S protein is in a trimeric form when preparing a COVID-19 serological diagnostic kit (Fig. 3).
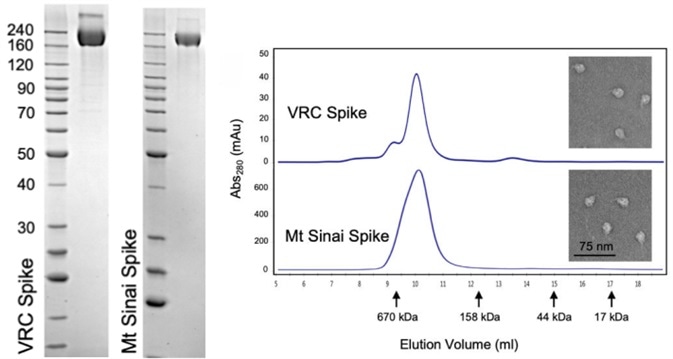
Figure 3. Verification of Spike trimer structure using SDS-PAGE, analytical size exclusion chromatography, and transmission electron microscopy. Image Credit: Carleen Klumpp-Thomas, et al. Standardization of enzyme-linked immunosorbent assays for serosurveys of the SARS-CoV-2 pandemic using clinical and at-home blood sampling, preprint at https://www.medrxiv.org/content/10.1101/2020.05.21.20109280v1.
ACROBiosystems has developed super stable trimeric S proteins with high purity, correct molecular weight and natural polymeric form rigorously verified by SDS-PAGE, SEC-MALS and NS-EM, respectively. The purity of (Cat. No. SPN-C52H9) was more than 90% verified by SDS-PAGE under reducing (R) condition. The molecular weight was around 550-660 kDa confirmed by SEC-MALS. The particles are similar in size and appearance to SARS-CoV-2 trimers reported in published literature verified by negative stain electron micrography.
References and Further Reading
- Wrapp D, et al., Cryo-EM structure of the 2019-nCoV spike prefusion conformation. Science, 2020 Mar 13;367(6483):1260-1263.
- R.J.G. Hulswit, et al., Coronavirus Spike Protein and Tropism Changes. Adv Virus Res. 2016; 96: 29–57.
- Song W, Gui M, Wang X, Xiang Y (2018) Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog 14(8): e1007236.
- Carleen Klumpp-Thomas, et al. Standardization of enzyme-linked immunosorbent assays for serosurveys of the SARS-CoV-2 pandemic using clinical and at-home blood sampling, preprint at https://www.medrxiv.org/content/10.1101/2020.05.21.20109280v1.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.