Researchers, biotech and pharma industry professionals have worked to rapidly develop vaccines and therapeutics following the outbreak of SARS-CoV-2. Breakthroughs and developments in research have successfully delivered a range of vaccines and antibody therapeutics to hospitals in under a year.
COVID-19 therapies that neutralize antibodies, thus blocking viral entry into host cells, have proven to be the most successful at reducing the disease’s severity and length.
The FDA has awarded two emergency use authorizations for monoclonal antibodies developed by Eli Lilly and Regeneron, while over ten new antibodies are currently undergoing clinical trials.
The immunoglobulin (IgG) antibodies being tested include variable heavy (VH) and variable light (VL) antigen-binding domains. This increases their size, therefore increasing the amounts required for therapy use while limiting delivery to intravenous (IV) drips.
Some mammals such as camels, alpacas, and similar species carry distinctive antibodies that include a VHH domain but no VL domain. When VHH domains occur by themselves, these are referred to as nanobodies. They have high stability and water solubility while offering improved tissue penetration and amenability to genetic engineering.
Nanobodies are also one-tenth of the size of an IgG antibody, effectively reducing manufacturing cost and making them suitable for delivery to patients via aerosolized particle in nasal spray.
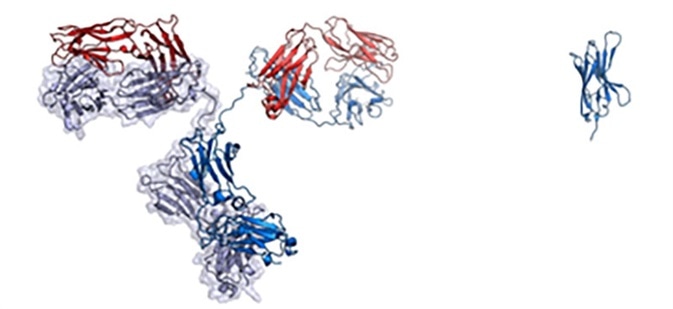
On the left is a full-sized IgG antibody while on the right is a nanobody. Image Credit: Walter and Manglik Labs/UCSF/HHMI
Scientists at the NIH published work outlining a nanobody, NIH-CoVnb-112, on December 22, 2020. Derived from a llama named Cormac, this nanobody was found to effectively neutralize SARS-CoV-2. The researchers also investigated the impact of aerosolization on nanobody activity.
Antibody development process and evaluation results
The development process and evaluation results of this antibody are outlined below.
The researchers began by immunizing an adult llama using the commercially available SARS-CoV-2 S1 protein (Cat.S1N-C5255). Next, they isolated the B-cells from the llama’s blood before amplifying the single heavy-chain variable domain in order to construct a 108 clones phage display library (Figure 1A).
The researchers screened nanobody clones employing the S protein RBD and ACE-2 competition method (Figure 1B). The human ACE2 (Cat. AC2-H52H8) was fixed to the radioimmunoassay tube and a premix of nanobody clones that had been incubated with biotinylated RBD (Cat. SPD-C82E9) was added.
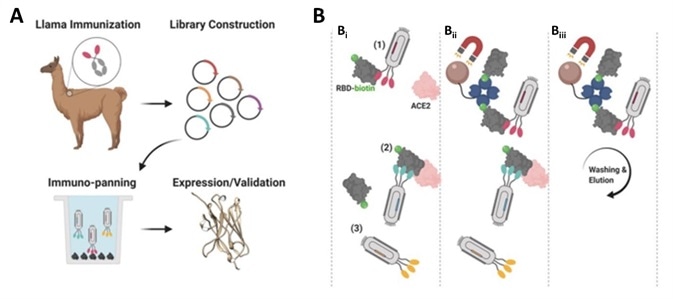
Figure 1. Nanobody development process. A) Llama immunization and identification of nanobody candidates. B) Selection of neutralizing nanobodies using three-step process. Image Credit: ACROBiosystems
Nanobodies binding to RBD and blocking RBD/ACE-2 interaction were removed from the solution using streptavidin-coated beads. Nanobodies that were not bound to the streptavidin beads were washed while neutralizing nanobodies bound to RBD were eluted and enriched.
Hundreds of nanobody clones were sequenced before 13 unique clones were isolated. These were numbered from NIH-CoVnb-101 to NIH-CoVnb-113. These nanobodies were overexpressed in E. coli and purified. Measurements of affinity between nanobody and RBD (Cat. SPD-C82E9) were taken using biolayer interferometry (BLI).
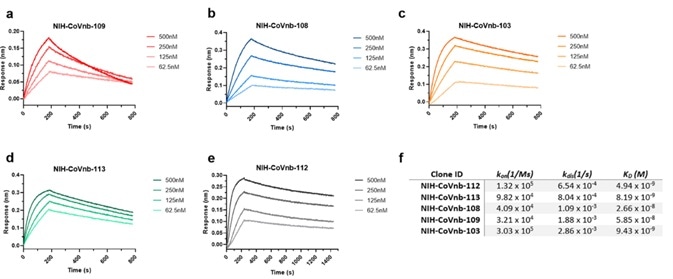
Figure 2. BLI analysis of RBD binding affinity of isolated nanobody clones. Image Credit: ACROBiosystems
Of the thirteen nanobodies measured, five exhibited the highest affinity in the nanomolar range (Figure 2). The NIH-CoVnb-112 nanobody exhibited the highest affinity to RBD with a KD = 4.9 nM (Figure 2).
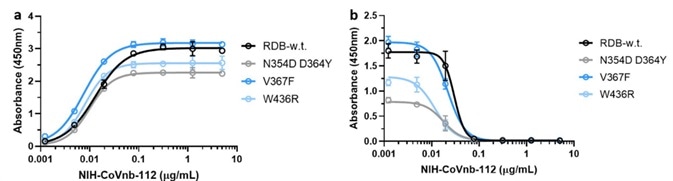
Figure 3. NIH-CoVnb-112 effectively suppresses ACE2/RBD binding. Image Credit: ACROBiosystems
In-vitro competitive experiments have demonstrated that screened nanobodies are able to block RBD/ACE2 binding, with NIH-CoVnb-112 maintaining the lowest EC50 value at 1.11 nM (Figure 3).
Mutations in the RBD region of the new coronavirus could help the virus to evade vaccine-induced immunity or reduced the efficacy of current neutralization antibodies. Because of this, the researchers evaluated the interaction of NIH-CoVnb-112 using three RBD mutants - N354D/D364Y, W436R, and V367F.
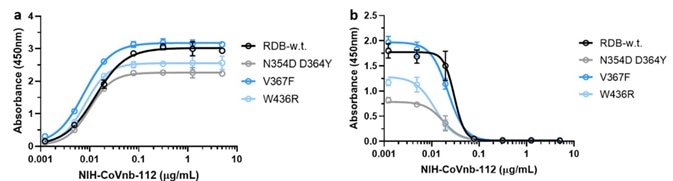
Figure 4. NIH-CoVnb-112 binds to RBD mutants and effectively inhibits ACE2/RBD mutant binding. Image Credit: ACROBiosystems
No notable variation in binding affinity (Figure 4a) was detected in comparison with wild-type RBD (Cat. SPD-C52H3). However, competition inhibition assays utilizing RBD coated plates that had been incubated with NIH-CoVnb-112 blocked ACE2 interaction demonstrated similar EC50 to each of the RBD mutants (Figure 4b).
This potentially indicates that NIH-CoVnb-112 can effectively suppress infection with new SARS-CoV-2, including these RBD mutations.
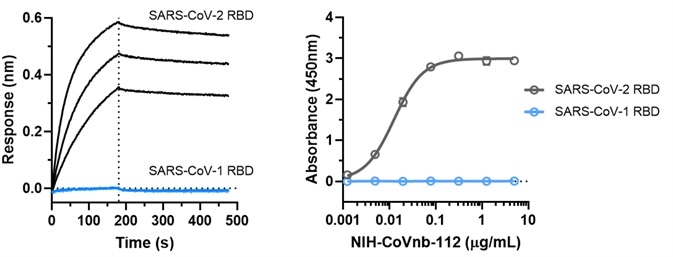
Figure 5. NIH-CoVnb-112 does not bind to SARS-CoV-1. Image Credit: ACROBiosystems
An assessment was also made of the binding affinity of NIH-CoVnb-112 to SARS-CoV-1 RBD. This was undertaken using BLI assay (Figure 5a) and ELISA (Figure 5b). Both assays revealed that NIH-CoVnb-112 was unable to bind to SARS-CoV-1 RBD.
Circular Dichroism (CD) spectroscopy was used to study the stability of NIH-CoVnb-112, determining that the nanobody’s structure began to unfold at 74.4°C. 73% of the nanobody was found to refold after the temperature had been lowered, therefore indicating that the nanobody benefits from high stability.
Figure 6. Aerosolized NIH-CoVnb-112 is structurally stable and can effectively block pseudotyped SARS-CoV-2 viral transduction. Image Credit: ACROBiosystems
Nanobodies can be aerosolized due to their low molecular weight and high stability. The researchers employed a commercial nebulizer to aerosolize NIH-CoVnb-112 (Figure 6a), using SDS-PAGE and size exclusion chromatography analysis to confirm that there was no aggregation or degradation of the nanobody following aerosolization (Figures 6b and 6c).
A fluorescence reporter assay utilizing S protein pseudotyped lentiviral infection was used (Figure 6d) to evaluate functional effects on the nanobody after nebulization. NIH-CoVnb-112 was found to be able to inhibit viral transduction both before and it had been aerosolized (Figure 6e).
These combined results confirm that the structure and activity of NIH-CoVnb-112 are unaffected by the aerosolization process.
Antibody potential
NIH-CoVnb-112 was the lead nanobody identified within this study and it was found to have good potential for development into a therapeutic against COVID-19.
Additional pre-clinical characterization is necessary before NIH-CoVnb-112 can be brought into a clinical trial, but ACROBiosystems is happy to have supplied key protein reagents at every step of the work.
These included the S1 protein used to immunize the llama, the biotin-RBD protein used for nanobody clone screening, and the ACE2 protein employed in the RBD/ACE2 interaction assays.
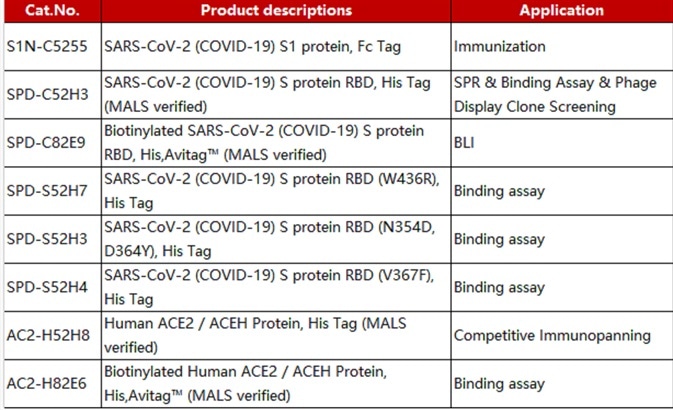
Table 1. Source: ACROBiosystems
Click to find out more SARS-CoV-2 related products
Prior to experiments being conducted, researchers confirmed the purity and activity of ACROBiosystem's SARS-CoV-2 products, returning adequate results (Figure 7a-c).
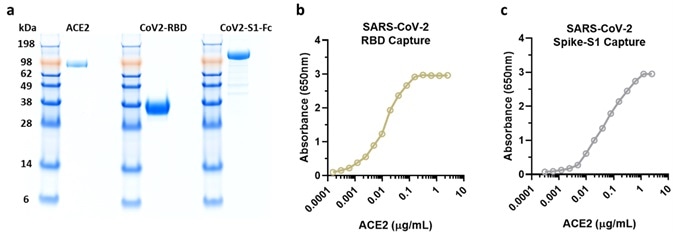
Figure 7. NIH researchers test ACRO protein purity and activity results. Image Credit: ACROBiosystems
ACROBiosystems will continue to assist in this vital work, as well as supporting wider research and development efforts in the fight against COVID-19 through the provision of high-quality protein and reagent products.
The company remains dedicated to helping advance vaccine development, targeted therapeutic drugs and diagnostics, by providing high-quality key reagents, target antigens, and related services.
Table 2. Source: ACROBiosystems
References and Further Reading
- Esparza TJ, Martin NP, Anderson GP, Goldman ER, Brody DL. High-affinity nanobodies block SARS-CoV-2 spike receptor binding domain interaction with human angiotensin converting enzyme. Sci Rep. 2020Dec 22; 10(1):22370. doi: 10.1038/s41598-020-79036-0. PMID: 33353972; PMCID: PMC7755911.
- Starr T N, Greaney A J, Hilton S K, et al. Deep mutational scanning of SARS-CoV-2 receptor binding domain reveals constraints on folding and ACE2 binding[J]. Cell, 2020, 182(5): 1295-1310. e20.
- Mulligan M J, Lyke K E, Kitchin N, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults[J]. Nature, 2020: 1-5.
- Rudberg A S, Havervall S, Månberg A, et al. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden[J]. Nature communications, 2020, 11(1): 1-8.
- Kondo T, Iwatani Y, Matsuoka K, et al. Antibody-like proteins that capture and neutralize SARS-CoV-2[J]. Science advances, 2020, 6(42): eabd3916.
- Ravichandran S, Coyle E M, Klenow L, et al. Antibody signature induced by SARS-CoV-2 spike protein immunogens in rabbits[J]. Science Translational Medicine, 2020.
- Hao W, Ma B, Li Z, et al. Binding of the SARS-CoV-2 Spike Protein to Glycans. BioRxiv preprint. doi: https://www.biorxiv.org/content/10.1101/2020.05.17.100537v1
- Pierce C A, Preston-Hurlburt P, Dai Y, et al. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients[J]. Science translational medicine, 2020, 12(564).
- Luetkens T, Metcalf R, Planelles V, et al. Successful transfer of anti–SARS-CoV-2 immunity using convalescent plasma in an MM patient with hypogammaglobulinemia and COVID-19[J]. Blood advances,2020, 4(19): 4864-4868.
- Ropa J, Cooper S, Capitano M L, et al. Human hematopoietic stem, progenitor, and immune cells respond ex vivo to SARS-CoV-2 spike protein[J]. Stem cell reviews and reports, 2020: 1-13.
- Lou Y, Zhao W, Wei H, et al. Cross-neutralization antibodies against SARS-CoV-2 and RBD mutations from convalescent patient antibody libraries. BioRxiv preprint. doi: https://doi.org/10.1101/2020.06.06.137513
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.