Few effective treatments exist to treat neurodegenerative diseases such as Alzheimer's disease (AD), Parkinson's disease (PD), and amyotrophic lateral sclerosis (ALS).1 Existing treatments focus on symptomatic relief but do not address the underlying pathology and, therefore, fail to slow or halt disease progression as a result.
The lack of effective treatment underscores the urgency to identify novel therapeutic targets relevant to the pathology of these conditions. Kinases are considered effective targets for drug development, especially with the notable success of kinase inhibitors in cancer treatment. Nonetheless, their capabilities in treating neurodegenerative diseases have not been thoroughly investigated, offering an exciting opportunity for future studies and the creation of new therapies.
The reasoning for targeting kinases in drug discovery for the central nervous system (CNS) stems from the crucial role of phosphorylation, facilitated by kinases, in regulating various cellular functions.
Many of these phosphorylation processes can become dysfunctional in disease states. In recent years, there has been a growing interest in the potential of brain-penetrant kinase inhibitors for treating neurodegenerative disorders.
The advancement of inhibitors such as glycogen synthase kinase-3β (GSK-3β) for Alzheimer's disease, leucine-rich repeat kinase 2 (LRRK2) for Parkinson's disease, and mitogen-activated protein kinase kinase kinase kinase (MAP4K) for amyotrophic lateral sclerosis (ALS) has shown promise. However, challenges such as specificity, overcoming the blood-brain barrier, and drug resistance persist.1-5
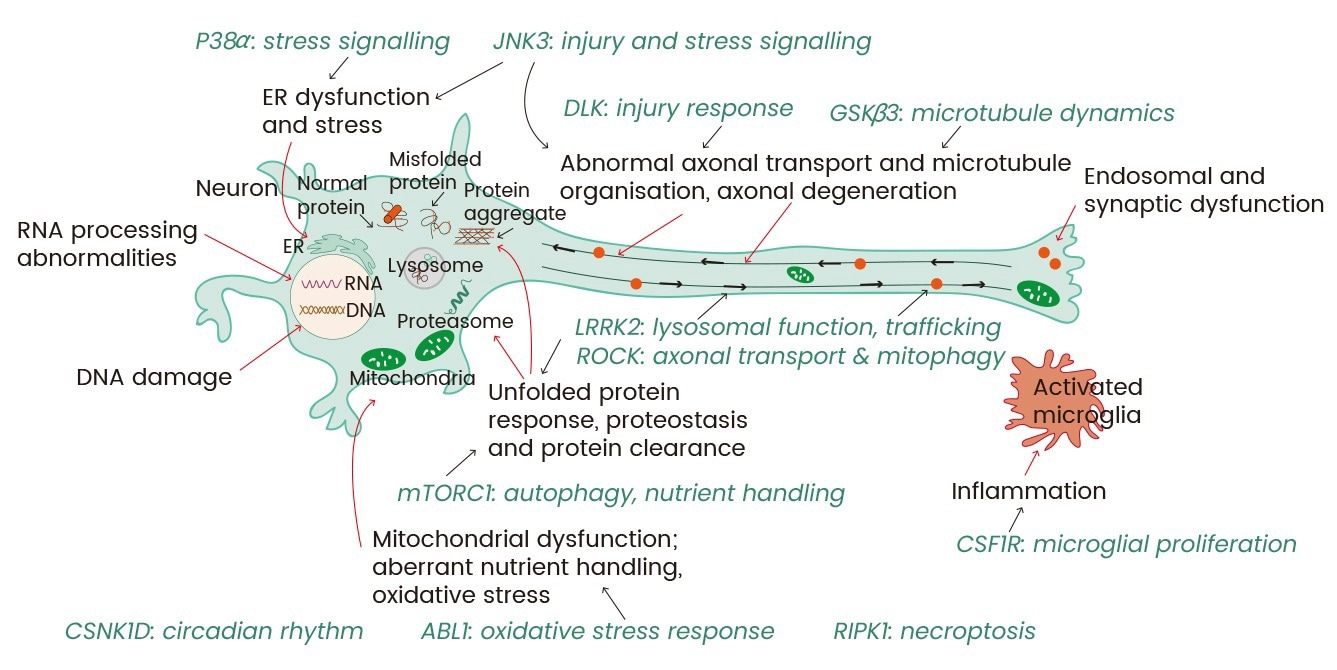
Figure 1. Promising kinase targets and converging pathways in neurodegenerative diseases. Image Credit: https://doi.org/10.3389/fnagi.2020.00242
The role of kinases in neurodegenerative diseases
Kinases play a crucial role in regulating signaling pathways within the brain, and dysfunction in their activity is linked to neurodegenerative conditions. In these diseases, specific kinases are often found to be either overactive or overexpressed in particular brain areas. This dysregulation typically leads to the hyperphosphorylation of proteins susceptible to aggregation, such as tau in Alzheimer's disease, α-synuclein in Parkinson's disease, and TDP-43 in amyotrophic lateral sclerosis.6
Abnormal kinase signaling can promote other harmful traits, such as neuroinflammation and neuronal cell death. On the other hand, the loss of function in certain kinases has been identified as a significant genetic factor contributing to the onset of various neurodegenerative diseases.
The primary pathological features of Alzheimer's disease (AD) are the extracellular amyloid beta (Aβ) plaques resulting from Aβ accumulation and the intracellular neurofibrillary tangles (NFTs) made up of abnormally phosphorylated tau protein. Increased activity of GSK-3β is directly associated with tau hyperphosphorylation and enhanced production, which leads to molecular dysfunctions, neuronal damage, and cognitive decline in AD.1-2
Fyn also emerges as an attractive therapeutic target for AD, as Aβ activates it via the cellular prion protein (PrPC) and interacts with tau, effectively linking the two major pathologies present in AD.7
Parkinson's disease (PD) is pathologically marked by the gradual loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc) and the buildup of intracellular α-synuclein, forming Lewy bodies.
Mutations in LRRK2 are the most prevalent genetic cause of familial PD and are a significant risk factor for sporadic cases.4,8 Mutations in PTEN-induced kinase 1 (PINK1) are linked to familial PD. Substantial evidence indicates that mitochondrial dysfunction plays a crucial role in the pathophysiology of PD.
Mutations in the autosomal dominant LRRK2 and autosomal recessive PINK1 are directly associated with mitochondrial impairment, underscoring their essential roles in the development of PD.
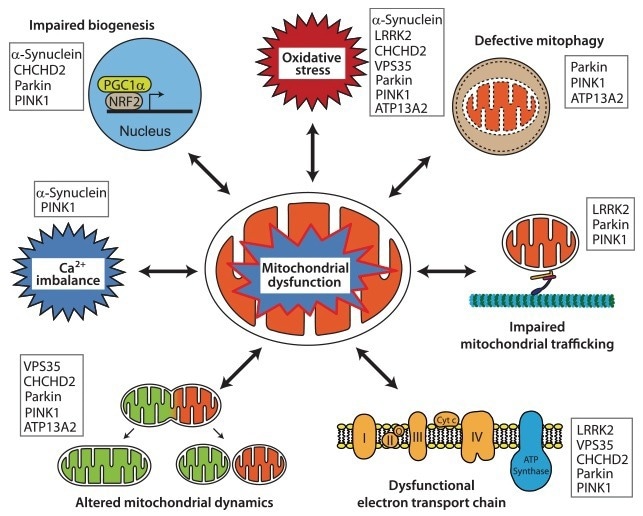
Figure 2. Representative pathways of mitochondrial dysfunction implicated in the pathophysiology of PD. The listed proteins contribute pathologically to the different pathways. Image Credit: https://doi.org/10.1007/s11910-018-0829-3
ALS is characterized by the gradual loss of muscle control due to motor neuron degeneration.
MAP4K4, a member of the STE20 family, has been identified as a key regulator of motor neuron degeneration in ALS.9,10 MAP4K4 inhibition improves neuron survival and prevents neurite degeneration under exogenous or endogenous stress conditions, suggesting that MAP4K4 is a druggable target for ALS therapeutics.
Development of kinase inhibitors for the treatment of AD, PD, and ALS
In AD: GSK-3β inhibitors have been examined extensively. GSK-3β is involved in tau hyperphosphorylation and Aβ formation, both critical pathological features of AD.
GSK-3β inhibitors, including lithium and other small molecules, demonstrate neuroprotective effects by reducing tau phosphorylation and Aβ levels.3,11 In addition, c-Jun N-terminal kinase (JNK) inhibitors are being considered for their role in preventing neuronal cell death.11
In PD: LRRK2 inhibitors are a major focus for treatment research.8 Mutations in LRRK2 are associated with familial and sporadic PD, and inhibiting its kinase activity has shown promising results in preclinical models.4,8 Many highly selective and potent LRRK2 inhibitors are currently being developed, with some progressing to clinical trials. LRRK2 inhibitors aim to reduce the pathological effects of mutated LRRK2, such as perturbed vesicular trafficking.12
In ALS: The development of MAP4K inhibitors has gained support. MAP4K inhibitors, such as Prosetin, show neuroprotective effects by protecting motor neurons from endoplasmic reticulum (ER) stress-induced apoptosis.5,9
Kinase inhibitors in preclinical and clinical trials for neurodegenerative diseases
Although multiple kinase inhibitors are currently undergoing clinical trials, none have been approved by the FDA specifically for neurodegenerative diseases.13 Recent years have highlighted the promising potential of these inhibitors in this field. These compounds are engineered to penetrate the blood-brain barrier and have demonstrated effectiveness in preclinical models, with continued efforts to progress them into clinical trials.
Table 1. Kinase inhibitors in preclinical and clinical trials for neurodegenerative diseases. Source: Sino Biological Inc.
| Disease |
Kinase |
Drug Name |
Mechanism of Action |
Development Stage |
| AD |
GSK-3β |
Tideglusib14 |
Inhibits GSK-3β activity,
reducing tau phosphorylation |
Clinical trials |
| Lithium14 |
Inhibits GSK-3β, used in
bipolar disorder and AD research |
Approved for bipolar
disorder, research for AD |
| AZD108015 |
Selective GSK-3β inhibitor |
Preclinical |
| JNK |
SP60012516 |
ATP-competitive
inhibitor of JNK |
Preclinical |
| AS60124517 |
Inhibits JNK
signaling pathway |
Preclinical |
| PD |
LRRK2 |
DNL151
(BIIB122)14 |
Small-molecule
kinase inhibitor |
Phase III |
| DNL20114 |
Small-molecule kinase inhibitor |
Phase I/II |
| ALS |
MAP4K |
URMC-09918 |
Inhibits MAP4K and
provides neuroprotection |
Preclinical |
| Prosetin9 |
Inhibits MAP4K and
protects motor neurons |
Preclinical |
| PF0626093319 |
Selective MAP4K
inhibitor |
Preclinical |
Challenges in developing kinase-targeted drugs
Creating kinase-targeted therapies for neurodegenerative diseases involves several distinct challenges. A significant concern is ensuring specificity. Since kinases share highly conserved ATP-binding sites, designing inhibitors exclusively targeting a specific kinase can be challenging. This lack of selectivity may result in off-target effects and potential toxicity.20
Moreover, the blood-brain barrier presents a considerable challenge. It limits the transport of numerous potential therapeutic agents into the brain, requiring the creation of drugs that can successfully navigate this barrier.1 The intricate nature of kinase signaling networks in the brain poses another challenge, as inhibiting just one kinase may not yield a therapeutic benefit.
Consequently, combination therapies are often necessary, adding complexity to treatment protocols.21 The emergence of drug resistance is also a significant concern, as cells may adapt to kinase inhibition via various mechanisms, such as mutations and the activation of alternative pathways.22
Addressing these challenges demands innovative drug design strategies, thorough preclinical evaluations, and well-structured clinical trials to create effective and safe kinase-targeted treatments for neurodegenerative diseases.
References and further reading
- Krahn, A.I., et al. (2020). Defining the Neural Kinome: Strategies and Opportunities for Small Molecule Drug Discovery to Target Neurodegenerative Diseases. ACS Chemical Neuroscience, 11(13), pp.1871–1886. https://doi.org/10.1021/acschemneuro.0c00176.
- Limantoro, J., Gervais, B. and Jane Carissa Sutedja (2023). Akt signaling pathway: a potential therapy for Alzheimer’s disease through glycogen synthase kinase 3 beta inhibition. The Egyptian Journal of Neurology, Psychiatry and Neurosurgery, 59(1). https://doi.org/10.1186/s41983-023-00751-2.
- Llorens-MarÃtin, M., et al. (2014). GSK-3β, a pivotal kinase in Alzheimer disease. Frontiers in Molecular Neuroscience, 7. https://doi.org/10.3389/fnmol.2014.00046.
- Taymans, J.-M., et al. (2023). MAP4K inhibition as a potential therapy for amyotrophic lateral sclerosis. Neural Regeneration Research, 19(8), pp.1639–1640. https://doi.org/10.4103/1673-5374.389639.
- Ma, S. and Zhang, C.-L. (2023). MAP4K inhibition as a potential therapy for amyotrophic lateral sclerosis. Neural Regeneration Research, 19(8), pp.1639–1640. https://doi.org/10.4103/1673-5374.389639.
- Axtman, A.D. (2021). Characterizing the role of the dark kinome in neurodegenerative disease – A mini review. Biochimica et Biophysica Acta (BBA) - General Subjects, 1865(12), pp.130014–130014. https://doi.org/10.1016/j.bbagen.2021.130014.
- Nygaard, H.B., van Dyck, C.H. and Strittmatter, S.M. (2014). Fyn kinase inhibition as a novel therapy for Alzheimer’s disease. Alzheimer’s Research & Therapy, 6(1), p.8. https://doi.org/10.1186/alzrt238.
- Cabezudo, D., Baekelandt, V. and Lobbestael, E. (2020). Multiple-Hit Hypothesis in Parkinson’s Disease: LRRK2 and Inflammation. Frontiers in Neuroscience, 14. https://doi.org/10.3389/fnins.2020.00376.
- Cobos, S.N. and Torrente, M.P. (2022). Epidrugs in Amyotrophic Lateral Sclerosis/Frontotemporal Dementia: Contextualizing a Role for Histone Kinase Inhibition in Neurodegenerative Disease. ACS Pharmacology & Translational Science, 5(2), pp.134–137. https://doi.org/10.1021/acsptsci.1c00265.
- Wu, C., Watts, M.E. and Rubin, L.L. (2019). MAP4K4 Activation Mediates Motor Neuron Degeneration in Amyotrophic Lateral Sclerosis. Cell Reports, 26(5), pp.1143-1156.e5. https://doi.org/10.1016/j.celrep.2019.01.019.
- Zhang, T., Byeong Mo Kim and Tae Ho Lee (2024). Death-associated protein kinase 1 as a therapeutic target for Alzheimer’s disease. Translational neurodegeneration, 13(1). https://doi.org/10.1186/s40035-023-00395-5.
- Lesniak, R.K., Nichols, R.J. and Montine, T.J. (2022). Development of mutation-selective LRRK2 kinase inhibitors as precision medicine for Parkinson’s disease and other diseases for which carriers are at increased risk. Frontiers in Neurology, 13. https://doi.org/10.3389/fneur.2022.1016040.
- Lui, A., et al. (2022). FDA-Approved Kinase Inhibitors in Preclinical and Clinical Trials for Neurological Disorders. Pharmaceuticals, 15(12), p.1546. https://doi.org/10.3390/ph15121546.
- U.S. National Library of Medicine (2025). Clinicaltrials.gov. (online) Clinicaltrials.gov. Available at: https://clinicaltrials.gov/.
- Arciniegas Ruiz, S.M. and Eldar-Finkelman, H. (2022). Glycogen Synthase Kinase-3 Inhibitors: Preclinical and Clinical Focus on CNS-A Decade Onward. Frontiers in Molecular Neuroscience, 14. https://doi.org/10.3389/fnmol.2021.792364.
- Rahman, M., et al. (2012). Intraperitoneal injection of JNK-specific inhibitor SP600125 inhibits the expression of presenilin-1 and Notch signaling in mouse brain without induction of apoptosis. Brain research, (online) 1448, pp.117–28. https://doi.org/10.1016/j.brainres.2012.01.066.
- Gibbons, G.S., et al. (2023). Identification of small molecules and related targets that modulate tau pathology in a seeded primary neuron model. The Journal of biological chemistry, (online) 299(7), p.104876. https://doi.org/10.1016/j.jbc.2023.104876.
- Bos, P.H., et al. (2019). Development of MAP4 Kinase Inhibitors as Motor Neuron-Protecting Agents. 26(12), pp.1703-1715.e37. https://doi.org/10.1016/j.chembiol.2019.10.005.
- Lasham, D.J., et al. (2023). Effects of MAP4K inhibition on neurite outgrowth. Molecular brain, (online) 16(1), p.79. https://doi.org/10.1186/s13041-023-01066-2.
- Benn, C.L. and Dawson, L.A. (2020). Clinically Precedented Protein Kinases: Rationale for Their Use in Neurodegenerative Disease. Frontiers in Aging Neuroscience, 12. https://doi.org/10.3389/fnagi.2020.00242.
- Li, S., et al. (2023). Signaling pathways in brain tumors and therapeutic interventions. Signal Transduction and Targeted Therapy, 8(1). https://doi.org/10.1038/s41392-022-01260-z.
- Grant, S.K. (2008). Therapeutic Protein Kinase Inhibitors. Cellular and Molecular Life Sciences, 66(7), pp.1163–1177. https://doi.org/10.1007/s00018-008-8539-7.
About Sino Biological Inc.
Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.