Stem cells, or SCs, have great potential as an unlimited source of tissues and cells for research and treatment of numerous diseases, and also for exploring early embryonic development.
Small molecules can be used at each stage of the stem cell workflow, and their use has numerous benefits in comparison to conventional methods: small molecules are versatile, animal-free, chemically-synthesized, and are cell permeable. Furthermore, the effects of small molecules are reversible and quick, and therefore their use can reduce the time required for reprogramming and differentiation protocols.
Reprogramming
Reprogramming is the regression of a specialized cell to a simpler state leading to cells that have stem-like properties, called induced pluripotent stem cells (iPSCs). Reprogramming of specialized human adult cells was first reported by Takahashi et al (2007), who expressed the transcription factors Oct3/4, Sox2, Klf4, and c-Myc (called the OSKM or Yamanaka factors) into adult human dermal fibroblasts through retroviral transduction. The ensuing iPSCs had analogous properties to ESCs and could differentiate into all three embryonic germ layers. Even now, similar methods are extensively used.
Small molecules can be used to improve the efficiency of reprogramming: Valproic acid (VPA) can improve reprogramming efficiency by Yamanaka factors by greater than 100 fold. Using a cocktail of growth factors and small molecules to reprogram cells removes the need for retroviral transduction and increases the reprogramming efficiency. The generation of iPSCs using only small molecules was first explained by Hou et al. (2013). The researchers found that a mixture of six compounds, VPA, CHIR 99021, RepSox, Tranylcypromine, Forskolin (these five compounds are collectively called VC6TF) and 3-Deazaneplanocin A, followed by culturing in 2i medium (see Self-Renewal section), could be used for reprogramming mouse somatic cells at 0.2% frequency (in comparison with 0.01% to 0.02% by the Takahashi technique).
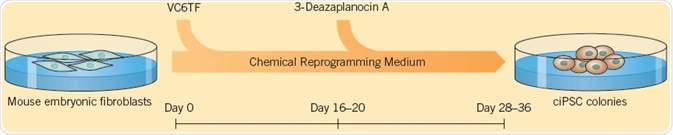
Fig 1. The generation of iPSCs using small molecules, explained by Hou et al. (2013). Image credit Tocris Bioscience
Zhao et al. (2018) modified this protocol, which resulted in a very effective technique for the generation of chemically induced PSCs (ciPSCs) from mouse embryonic fibroblasts (MEFs). The three-stage process uses 12 compounds (including VC6TF), in addition to the growth factors, bFGF, and LIF for generating ciPSCs in 16 to 20 days compared with 30 days using Yamanaka factors; this kind of chemical reprogramming shows potential for generating cells for disease modeling and cell therapy.
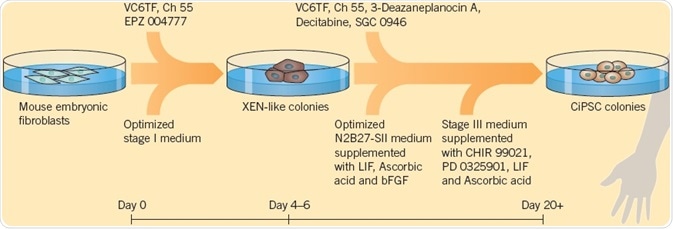
Fig 2. The modified protocol by Zhao et al. (2018), used to generate ciPSCs. Image credit Tocris Bioscience
Self-Renewal and Proliferation
Stem cells renew themselves and proliferate through the division of a parent cell into two identical daughter cells. Traditional culture techniques for PSCs need serum products, ‘feeder’ cells, and growth factors, such as bFGF and LIF. In recent times, chemically-defined serum-free media have been developed to substitute the need for serum products and feeder cells, and small molecules can be applied to maintain self-renewal of stem cells.
Chen et al. (2006) were the first to demonstrate that a small molecule that hinders differentiation, SC 1, also called Pluripotin, can be used to preserve pluripotency in the absence of growth factors. Ying et al. (2008) consequently found a combination of two small molecules (called 2i) — the GSK-3 inhibitor CHIR 99021 and the MEK inhibitor PD 0325901, which can maintain the self-renewal of ESCs. Compared to conventional SC culture techniques, ESCs grown in 2i medium show lower levels of spontaneous differentiation. Moreover, 2i can be used to save cultures that have started to decay. The ROCK inhibitor Y-27632 also plays a significant role in the maintenance of stem cells as it improves cloning efficiency of ESCs without affecting pluripotency, allowing the survival of stem cells in culture over a long period (Watanabe et al, 2007).
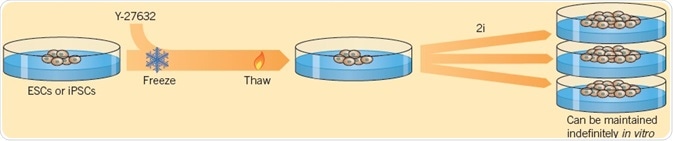
Fig 3. Using two small molecules, Ying et al. (2008) demonstrated the self-renewal of ESCs. Image credit Tocris Bioscience
Storage
Cryopreservation is used for storing cells, including stem cells for in vitro culture, but the survival rate of cells can be reduced after freeze-thawing. Li et al. (2008) discovered that when hESCs are treated with the ROCK inhibitor Y-27632 before freezing, the viability of cells is considerably increased after thawing. Moreover, Y-27632 enhances the survival of cells that have been differentiated from PSCs, facilitating their application in therapy and disease modeling.
Differentiation
Stem cells can be classified into various types of cells with a range of potential uses, including toxicity testing, drug screening, and disease modeling. They also show potential for treating conditions such as diabetes, traumatic injury, and neurodegenerative diseases, to name a few. Small molecules are multipurpose tools to control the fate of stem cells and direct differentiation toward particular cell types.
A technique for deriving functional human pancreatic β-cells from hPSCs developed by Pagliuca et al. (2014) provides the possibility of a new method for treating diabetes. Their six-stage protocol employs a combination of 11 small molecules and proteins (CHIR 99021, LDN 193189, Retinoic Acid, Phorbol 12,13-dibutyrate, SANT-1, Activin A, T3, Compound E, Betacellulin, RepSox, Heparin, and KGF) and generates functional β-cells in 28 days, which when administered into diabetic mice are found to ameliorate hyperglycemia.
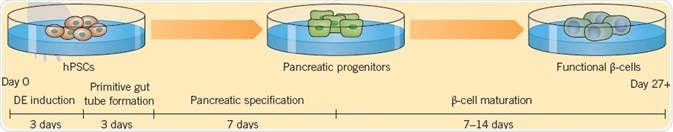
Fig 4. Pagliuca et al. (2014) derived functional human pancreatic β-cells from hPSCs. Image credit Tocris Bioscience
For deriving cortical neurons from hiPSCs, Qi et al. (2017) have designed a protocol using a cocktail of six small molecules. When transplanted into postnatal mouse cortex at day 8 of differentiation, the resultant neurons are functional and establish long-term connections. Additionally, cells remaining in culture show functional electrophysiological properties by day 16.

Fig 5. Using a protocol of six small molecules, Qi et al. (2017) created cortical neurons from hiPSCs. Image credit Tocris Bioscience
Cells can also be reprogrammed directly from one specialized cell type to another by a process called direct-lineage reprogramming or transdifferentiation. Cao et al. (2016) explained a technique for converting human fibroblasts into cardiomyocytes using a combination of nine small molecules: Y-27632, A83-01, SC1, CHIR 99021, OAC-2, A 8351, SU 16f, BIX 01294, and JNJ 10198409 (9C). The 9C-treated cells were consequently cultured in cardiac induction medium and transplanted into mice where they are changed into cardiomyocyte-like cells.
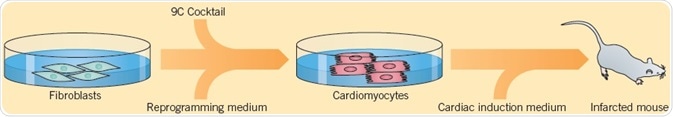
Fig 6. Cao et al. (2016) displayed a technique used for converting human fibroblasts into cardiomyocytes. Image credit Tocris Bioscience
Verification
The characterization of differentiated cells is an essential step prior to using the cells in assays or therapy. Western blotting or immunohistochemistry — using antibodies against particular markers exhibited by the target cell — are methods extensively used to validate the identity of differentiated cells, just like quantitative RT-PCR, which is used for analyzing gene expression. Small molecules have a vital role to play in the verification of cell function, by electrophysiological or pharmacological methods.
After the conversion of fibroblasts into cardiomyocytes, Cao et al (2016) determined the presence of genes that take part in cardiomyocyte function. Electrophysiological analysis of the cells showed ventricular-like action potentials. The scientists then studied the effect that small molecules have on action potential firing and discovered that the non-selective β-adrenoceptor agonist Isoprenaline, as well as Caffeine, elevated the firing rate, while the muscarinic agonist Carbachol decelerated the firing rate, aiding to determine the identity of the differentiated cells as functioning cardiomyocytes.
Telezhkin et al. (2016) explained the verification of functional neurons differentiated from iPSCs. Electrophysiological analysis showed spontaneous action potential firing, which was eliminated by the administration of Tetrodoxin, signifying the presence of functioning sodium channels. Application of GABA resulted in inhibitory postsynaptic currents that could be hindered by Bicuculline, indicative of the development of a functioning neural network. Calcium imaging using FURA-2AM showed Ca2+-influx in response to application of neurotransmitters Glycine, Glutamate, or GABA. Such methods offer proof for the presence of mature functioning cells.
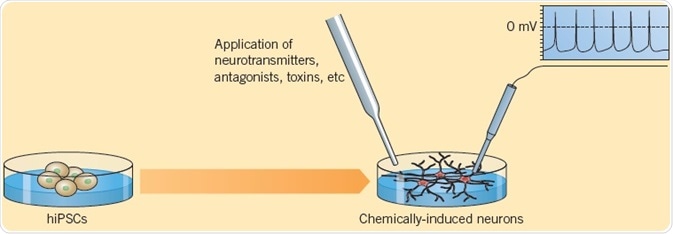
Fig 7. Telezhkin et al. (2016) explained the verification of functional neurons differentiated from iPSCs. Image credit Tocris Bioscience
References
- Cao, N et al. (2016) Science 352 6290
- Chen, S et al. (2006) Proc.Natl.Acad.Sci.USA 103 17266
- Hou, P et al. (2013) Science 341 651
- Ichikawa, H et al. (2011) Cryo Letters 32 516
- Li, X et al. (2008) Stem Cells Dev. 17 1079
- Pagliuca, FW et al. (2014) Cell 159 428
- Takahashi, K et al. (2007) Cell 131 861
- Tamm, C et al. (2013) PLoS One 8 e81156
- Telezhkin, V et al. (2016) Am.J.Physiol.Cell Physiol. 310 C520
- Watanabe, K et al. (2007) Nat.Biotechnol. 25 681
- Wilson, HK et al. (2016) Tissue Eng. Part C, Methods 22 1085
- Ying, QL et al. (2008) Nature 453 519
- Zhao, T et al. (2018) Cell Stem Cell 23 31
About Tocris Bioscience
Tocris Bioscience is your trusted supplier of high-performance life science reagents, including receptor agonists & antagonists, enzyme inhibitors, ion channel modulators, fluorescent probes & dyes, and compound libraries. Our catalog consists of over 4,500 research tools, covering over 400 protein targets enabling you to investigate and modulate the activity of numerous signaling pathways and physiological processes.
We have been working with scientists for over 30 years to provide the life science community with research standards, as well as novel and innovative research tools. We understand the need for researchers to trust their research reagents, which is why we are committed to supplying our customers with the highest quality products available, so you can publish with confidence.
Tocris is part of the protein sciences division of Bio-Techne, which also includes the best in class brands R&D Systems, Novus Biologicals, ProteinSimple, and Advanced Cell Diagnostics. Bio-Techne has united these brands to provide researchers with a full portfolio of research reagents, assays, and protein platforms. For more information on Bio-Techne and its brands, please visit bio-techne.com.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.